Androgens Regulate Human Female Reproductive Ageing and Meiotic Fidelity
Judith Helen Ford
Judith Helen Ford*
Lecturer in Research Education, Teaching Innovation Unit, University of South Australia, Adelaide, SA Australia
- *Corresponding Author:
- Judith Helen Ford
Lecturer in Research Education, Teaching Innovation Unit
University of South Australia, Adelaide, SA Australia
Tel: + 61883025556
E-mail: Judy.Ford@unisa.edu.au
Received date: January 10, 2017; Accepted date: January 28, 2017; Published date: February 03, 2017
Citation: Ford JH (2017) Androgens Regulate Human Female Reproductive Ageing and Meiotic Fidelity. J Rep Endo Infert 2:20. DOI: 10.4172/2476-2008.100020
Copyright: © 2017 Ford JH. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To develop a model that explains the complex relationships that ultimately lead to increased rates of follicle loss and high rates of trisomy in the oocytes of normal women aged about 38 and older.
Design: Review of the key published findings that contribute to the diverse literature affecting this phenomenon.
Results: In humans and higher primates, androgen synthesis is optimised by the specialisation of the andrenal zona reticularis (ZR). Whilst this allows very high rates of synthesis of DHEA and related androgens in early post-pubertal life, it compromises the quality of oocyte maturation in the later years of female fertility and is linked to the evolution of menopause. Data from genetics, endocrinology and lifestyle studies of ovarian physiology provide substantial evidence for a model of androgen regulation of human female reproductive ageing.
Conclusions: Human female reproductive ageing and loss of meiotic fidelity results from a chain of events initiated in the ZR. Because of inadequate synthesis of DHEA by the lowered number of cells ZR in the mid-thirties age-group, there is lowered synthesis of inhibin B and subsequent reduction in DHEA synthesis by the ovarian theca cells. Low DHEA lowers both ovarian androstenedione and PPAR alpha. The latter reduces both fat metabolism and transport which in turn causes increased rates of apoptosis (lowering follicle pool size) and other cellular changes in oocytes that induce high rates of chromosome errors in meiosis.
Short Communication
The phenomena of accelerated ageing and menopause in human female reproduction
Despite the vast amount of research into human fertility and the development of highly refined and exquisite technologies used to treat both male and female infertility, the intrinsic mechanism underlying female reproductive ageing is yet to be fully elucidated. This paper attempts to outline the key parameters underlying female reproductive ageing and puts forward a model that can be tested and refined by further research.
Human females differ from most other animals in having an extended post-fertility lifespan. Even most of the evolutionarily closest primates die before their reproductive life ceases [1] whilst in humans the secondary non-fertile lifespan often exceeds several decades. A key characteristic of the approaching end of human female fertility is a distinctive phase in which there is an accelerated rate of decline in oocyte numbers and loss of meiotic fidelity. This phenomenon was first noted through the births of infants with Down’s syndrome (trisomy 21) [2] and through age-related increases in spontaneous abortions with trisomic karyotypes [3]. Cessation of normal fertility precedes menopause by about seven to ten years during which time, menstrual cycles gradually change in length and regularity [4]. Most animals do not exhibit similar patterns of reproductive ageing and to date, the cynomolgus macaque is the only other primate proven to have a naturally occurring menopause [5] although menopause is suspected in some other primates [6].
The same pattern of reproductive ageing is seen in all human ethnic groups and apart from tuberculosis, cancer and hysterectomy, which are all associated with premature ovarian failure (POF), the only common lifestyle factor associated with early menopause is smoking.
The effect of smoking on menopause has been thoroughly assessed and it has been found to be an independent factor associated with both POF and early menopause [7] and with early menopause in many large studies. There is an anomaly with smoking, however, and that is that smokers have fewer trisomic spontaneous abortions than non-smokers in the later years of their fertility even though the length of fertility is compromised [8].
The evolution of the adrenal reticularis, adrenarche and menopause
The restriction of menopause to humans and a few closely related higher primates suggests that the explanation for the phenomenon of accelerated reproductive ageing prior to menopause lies within the genes associated with the evolution of menopause. These genes primarily affect steroid metabolism and the concordant specialisation of the adrenal zona reticularis (ZR), a region in the innermost part of the adrenal cortex. At the stage of evolution of humans and cynomolgus macaques the ZR is devoted to the synthesis of dehyroepiandrosterone (DHEA) [9] and its specialisation allows the production of milligram quantities of DHEA, which in humans is produced in maximum concentrations at about age 20 [10]. The most well recognised function of DHEA is that it is the major precursor of androgens and oestrogens. DHEA itself has a short half-life and is quickly converted into the sulphated derivative DHEAS, its major circulatory form. In androgen synthesis, DHEA is converted into androstenedione, which is converted into estrone or testosterone.
Specialization of the ZR has been accompanied by the evolution of a physiological developmental stage called the adrenarche. In humans adrenarche precedes puberty by a few years and involves migration of cells into the ZR and commencement of DHEA synthesis. Concordant with the evolution of the ZR is an evolutionary change in the gene sequence of the CYP17A1 gene (the gene that produces the cytochrome P45017 enzyme). Gene sequencing of the CYP17A1 gene shows most similarity between humans and those primates where P450c17 and CytB5 are co-localised in three specialised androgen tissues: namely the ZR in both males and females, the Sertoli cells in males and theca (ovarian) cells in females [11]. In embryogenesis, the three cell types arise from the mesonephros and migrate to either the gonads or ZR [12]. This specialisation of the ZR in higher primates appears to be essential to the occurrence of both the adrenarche and the menopause [13] but there is still much to learn about the ZR and how its dysregulation might be underlying the growing problems of premature adrenarche [14].
Ageing of the adrenal reticularis and its effects on serum DHEA-S levels
Ageing of cells in the human ZR occurs relatively early in life. No telomerase reverse transcriptase (hTERT) activity has been detected in any cells of the adrenal cortex, telomere length is reduced and the cells’ replicative ability has reduced to almost zero by age 40 in both human males and females [15]. The loss of cells in the adrenal cortex seems mostly to affect the ZR, which at about age 40 is much reduced in size [16,17]. A dramatic decline in DHEAS and Androstenedione occurs between the early 20’s and mid 30’s age groups followed by a steady decline over the following decades with a slowing of the rate of decline in the sixth decade [18].
DHEA in the ovary
Although the ZR plays the major role in steroid synthesis before age 40, ovarian theca cells also synthesise DHEA. Moreover, given that the theca cells develop from the ovarian stroma and surround early secondary follicles before the antral stage, it might be expected that the theca cells would normally provide all the DHEA required for the early maturation of the follicle [19].
Androgens are involved in the recruitment of resting primordial follicles into the actively growing pool [20] and recruitment declines with ageing: the number of ‘type B follicles’, in which ‘the oocytes are surrounded by flattened granulosa cells’ are greatly reduced with age (r=-0.87, p<0.001) [21]. Conversely, treatment with DHEA for four months can increase follicle numbers and quality [22] and is marked by increases in Anti-Müllarian Hormone (AMH) [23]. Not all published studies show success with DHEA treatment but it is likely that this is because of study design. Two poor designs are common: in the first DHEA is administered for less than four months while in the second, DHEA is administered to study participants having premature ovarian failure (POF) of undefined cause.
No in depth studies of human theca cell DHEA synthesis in normal ageing have yet been reported, however in fertile women studied after autopsy, the P450c17 protein was expressed by theca cells of secondary follicles greater than 250 μm diameter. DHEA was not present in follicles nor stroma of perimenopausal women but was present in low levels in the ovarian stroma cells of postmenopausal women [24]. Furthermore, in histological observations of theca P450c17 and CytB5 in the cynomolgus macaques, the quantity of DHEA synthesised by theca cells was reduced with ageing [25].
That follicle recruitment and AMH can be increased by externally provided DHEA suggests that the observed age-reduction in both follicle recruitment and androgen synthesis by theca cells could also be affected by changes external to the ovary, notably those occurring in the ZR. There are no studies yet that directly test this concept.
Inhibin B and androgen synthesis
The alpha subunit of Inhibin B is the major stimulator of DHEA synthesis and is usually expressed by both the ZR cells [26] and the ovarian granulosa cells [27]. The loss of ZR cells with age can account for the lowered secretion of inhibin B observed with ageing [28] and since Inhibin B is a massive up-regulator of theca cell androgen synthesis [29,30], there will be lowered stimulation of DHEA synthesis in the remaining follicles. All the studies performed to date identify the importance of inhibin B in androgen synthesis and its importance in reproductive ageing is further emphasised by the finding that genetic variants of the INH alpha gene are highly significantly associated with premature ovarian failure [31].
Model for female reproductive ageing involving the ovary and the adrenal zona reticularis
Recent research has focussed attention on the use of DHEA in the possible treatment of age-related reduction in fertility [32] and I have previously published a model that explains how lowered levels of ovarian DHEA with ageing could account for both the reduction in the size of the ovarian pool and the reduction in oocyte quality and susceptibility to meiotic error, especially trisomy [33]. Correcting androgen levels at an early enough stage in oocyte maturation should overcome the cascade of deleterious events and abnormal cell division in older women with otherwise normal fertility.
A revision of my model of human reproductive ageing is presented here. This is similar to the previous model published [33] but has been modified slightly to account for androgen-related research not included in the previous publication. The figure outlines a model for the control of female reproductive ageing and menopause timing by the ovary and the ZR that is primarily driven by the evolution of the specialisation of the ZR in homo sapiens and the higher primates.
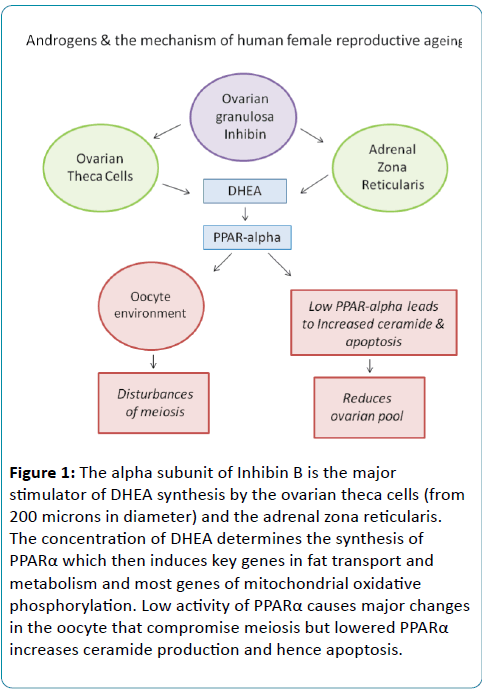
Summary of the model (Figure 1):
Figure 1: The alpha subunit of Inhibin B is the major stimulator of DHEA synthesis by the ovarian theca cells (from 200 microns in diameter) and the adrenal zona reticularis. The concentration of DHEA determines the synthesis of PPARα which then induces key genes in fat transport and metabolism and most genes of mitochondrial oxidative phosphorylation. Low activity of PPARα causes major changes in the oocyte that compromise meiosis but lowered PPARα increases ceramide production and hence apoptosis.
In normal female fertility, DHEA is synthesised by both the ovarian theca cells and the adrenal zona reticularis (ZR). The synthesis of DHEA is stimulated by the alpha sub-unit of Inhibin B, which is synthesised by both the ZR cells and the ovarian granulosa cells.
The size of the ZR is markedly reduced with age. The loss of ZR cells lowers the synthesis of DHEA and Inhibin B.
Adequate DHEA synthesis is critical for female fertility in at least two ways. First, it is a precursor of androstenedione and plays a major role in determining the hormonal environment of the ovary. Second, it is an essential ligand for PPAR alpha which in turn is the key promoter of genes involved in fat metabolism and fat transport. Fat metabolism and transport are in turn critical to preserving normal cytoplasmic function, especially mitochondrial structure and function, which are critical to normal meiotic and mitotic cell division.
If PPAR alpha is low, the reduced conversion of stearic acid to oleic acid, through lowered delta-9 desaturase in the Omega 9 fatty acid pathway, promotes conditions that lead to increased ceramide synthesis. Apoptosis is induced by ceramide and the rate of follicle loss is accelerated.
Diverse research that supports the proposed mechanism of human reproductive ageing
Research in four somewhat disparate areas namely polycystic ovarian syndrome, use of the contraceptive pill, unilateral ovariectomy and smoking, provides valuable insight into the mechanism of reproductive ageing. In each, DHEA synthesis is altered and the fertility of older women is significantly affected.
Polycystic ovarian syndrome and ovarian ageing
Polycystic ovarian syndrome (PCOS) is a common condition that is characterised by polycystic ovaries and is accompanied by hyperandrogenisation and infertility. This relatively common condition with a prevalence of about 6.5% in women of normal fertile age [34]. Hyperandrogenism is a key characteristic of PCOS and the condition seems to have at least three different aetiologies. PCOS is most commonly associated with overproduction of androgens by the ovarian theca cells [35,36], and by the adrenal ZR in about 25-30% of affected women [37]. PCOS can also be induced by metabolic changes in severely overweight or obese women and it has been suggested that this condition, in which women are at considerable metabolic risk should be renamed [38].
In high androgen-synthesising forms of PCOS excessive androgen synthesis overstimulates the follicles as the term PCOS describes. However, as the women age and at the stage where normal women are starting to experience inadequate ovarian androgen stimulation, about 30% of older women with PCOS will experience normal menstrual cycles and normal fertility [39]. Like normal women in the late thirties and early forties, the DHEA levels in these PCOS women greatly decline from their peak levels but in older women with PCOS, the DHEA levels might now be within the normal fertile range and so normal conceptions are possible [39]. In a study of older women with PCOS, 20 women with regular periods had mean Inhibin B levels of 82 ng/L.
This is higher than the levels found in non-PCOS older women and closer to the same range as normal young women (Klein et al., 2004) and gives strong support to the argument that female fertility is ultimately determined by androgen levels.
The contraceptive pill and ovarian ageing
In women aged 30 or older with normal fertility, the rate of spontaneous abortion was decreased by approximately 50% in those who took the pill for nine years or more [15]. Other studies have also found that women who take the pill for long periods have reduced rates of reproductive ageing, prolongation of their fertile period and later menopause e.g [7,40].
Oral contraceptive use is associated with low levels of DHEA. Highly significant reductions (an average of 35%) in plasma DHEA, DHEAS and androstenedione were found in young women (mean age about 25) when they took any one of three contraceptive pills for a period of at least six months [41].
There is no research to date that investigates how the lowered levels of DHEA whilst taking the pill might relate to the extension of fertility but the finding lends weight to the proposal that the ZR is involved in the regulation of the timing of reproductive ageing.
Unilateral ovariectomy and ageing
Unilateral ovariectomy causes only a relatively minor acceleration of ‘reproductive aging’ in humans [42] whereas in mice, it causes a significant acceleration of reproductive ageing [43]. The difference in these results suggests that at least some of the cause of human reproductive ageing is external to the ovary.
Smoking and ovarian ageing
Smokers have an earlier than average age at menopause and a greater chance of premature ovarian failure. The ovarian failure could be caused by many different mechanisms but is at least partially attributable to the generation of oxygen radicals, lowered follicular beta-carotene and destruction of oocytes [44,45]. However, despite their earlier menopause, smokers do not show an acceleration of age-related trisomic conceptions in the later phase of their reproductive life [8,46]. This ‘improved quality’ of the remaining follicles might be explained by the involvement of the ZR because amongst its other physiological effects, smoking elevates the synthesis of DHEA by the ZR and relatively high levels of DHEA persist until infertility [47].
Further research requirements
There are still many gaps in knowledge in this important area of research. To date, the literature has been confused both by the use of unsuitable animal models that do not have the same specialisation of the ZR and by lack of critical attention to differences between the human subjects used in observational and experimental studies. Premature ovarian failure in younger women is often caused by genes that affect the biochemical pathways involved in the normal responses to androgens [35] and such subjects should not be included in the same trials as women with age-reduced fertility. The role of androgen depletion in age-related infertility in normal women, and its possible treatment by DHEA or other androgens has been greatly compromised by this lack of understanding.
Conclusions and Clinical implications
Both the rapid decline in the follicular pool and the loss of meiotic fidelity with ageing are explained by lowered androgens and the secondary effects on fat metabolism. Consequently, for women whose reproduction has been normal until they reach the age of about 36, it should be possible to restore normal fertility with DHEA treatment for a period of four months prior to desired conception, without the need for other interventions.
There is clearly a limit to how far normal fertility can be extended and no doubt this will be established by new research. To date there has been relatively little research into the ZR and its role in reproductive ageing. Further research into PCOS is likely to be helpful in identifying genes in this key metabolic pathway.
References
- Alberts SC, Altmann J, Brockman DK, Cords M, Fedigan LM, et al. (2013) Reproductive aging patterns in primates reveal that humans are distinct. Proc Natl Acad Sci U S A 110: 13440-13445.
- Lamson SH, Hook EB (1980) A simple function for maternal-age-specific rates of Down syndrome in the 20-to-49-year age range and its biological implications. Am J Hum Genet 32: 743-753.
- Boué J, Boué A, Lazar P (2013) Retrospective and prospective epidemiological studies of 1500 karyotyped spontaneous human abortions. 1975. Birth Defects Res A Clin Mol Teratol 97: 471-486.
- te Velde ER, Pearson PL (2002) The variability of female reproductive ageing. Hum Reprod Update 8: 141-154.
- Kavanagh K, Koudy WJ, Wagner JD (2005) Naturally occurring menopause in cynomolgus monkeys: changes in hormone, lipid, and carbohydrate measures with hormonal status. J Med Primatol 34: 171-177.
- Walker RS, Gurven M, Burger O, Hamilton MJ (2008) The trade-off between number and size of offspring in humans and other primates. Proc Biol Sci 275: 827-833.
- Chang SH, Kim CS, Lee KS, Kim H, Yim SV, et al. (2007) Premenopausal factors influencing premature ovarian failure and early menopause. Maturitas 58: 19-30.
- Kline J, Levin B, Kinney A, Stein Z, Susser M et al. (1995) Cigarette smoking and spontaneous abortion of known karyotype. Precise data but uncertain inferences. Am J Epidemiol 141: 417-427.
- Pattison JC, Abbott DH, Saltzman W, Conley AJ, Bird IM (2009) Plasticity of the zona reticularis in the adult marmoset adrenal cortex: voyages of discovery in the New World. J Endocrinol 203: 313-326.
- Labrie F, Luu-The V, Bélanger A, Lin SX, Simard J, et al. (2005) Is dehydroepiandrosterone a hormone? J Endocrinol 187: 169-196.
- Dharia S, Slane A, Jian M, Conner M, Conley AJ, et al. (2004) Colocalization of P450c17 and cytochrome b5 in androgen-synthesizing tissues of the human. Biol Reprod 71: 83-88.
- Hammer GD, Parker KL, Schimmer BP (2005) Minireview: transcriptional regulation of adrenocortical development. Endocrinology 146: 1018-1024.
- Auchus RJ, Rainey WE (2004) Adrenarche - physiology, biochemistry and human disease. Clin Endocrinol (Oxf) 60: 288-296.
- Bird IM (2012) In the zone: understanding zona reticularis function and its transformation by adrenarche. J Endocrinol 214: 109-111.
- Yang L, Suwa T, Wright WE, Shay JW, Hornsby PJ (2001) Telomere shortening and decline in replicative potential as a function of donor age in human adrenocortical cells. Mech Ageing Dev 122: 1685-1694.
- Hornsby PJ (2002) Aging of the human adrenal cortex. Ageing Res Rev 1: 229-242.
- Hornsby PJ (1995) Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann N Y Acad Sci 774: 29-46.
- Orentreich N, Brind JL, Rizer RL, Vogelman JH (1994) Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab 59: 551-555.
- Wickenheisser JK, Nelson-DeGrave VL, McAllister JM (2006) Human ovarian theca cells in culture. Trends Endocrinol Metab 17: 65-71.
- Vendola K, Zhou J, Wang J, Bondy CA (1999) Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum Reprod 14: 2328-2332.
- Gougeon A, Chainy GB (1987) Morphometric studies of small follicles in ovaries of women at different ages. J Reprod Fertil 81: 433-442.
- Zangmo R, Singh N2, Kumar S1, Vanamail P1, Tiwari A1 (2014) Role of dehydroepiandrosterone in improving oocyte and embryo quality in IVF cycles. Reprod Biomed Online 28: 743-747.
- Gleicher N, Weghofer A, Barad DH (2010) Anti-Müllerian hormone (AMH) defines, independent of age, low versus good live-birth chances in women with severely diminished ovarian reserve. Fertil Steril 94: 2824-2827.
- Inkster SE, Brodie AM (1991) Expression of aromatase cytochrome P-450 in premenopausal and postmenopausal human ovaries: an immunocytochemical study. J Clin Endocrinol Metab 73: 717-726.
- Ethun KF, Wood CE, Parker CR, Kaplan JR, Chen H, et al. (2010) Effect of ovarian aging on androgen biosynthesis in a cynomolgus macaque model. Climacteric 15: 82-92.
- Vänttinen T, Liu J, Kuulasmaa T, Kivinen P, Voutilainen R (2003) Expression of activin/inhibin signaling components in the human adrenal gland and the effects of activins and inhibins on adrenocortical steroidogenesis and apoptosis. J Endocrinol 178: 479-489.
- Welt CK, Smith ZA, Pauler DK, Hall JE (2001) Differential regulation of inhibin A and inhibin B by luteinizing hormone, follicle-stimulating hormone, and stage of follicle development. J Clin Endocrinol Metab 86: 2531-2537.
- Welt CK, McNicholl DJ, Taylor AE, Hall JE (1999) Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab 84: 105-111.
- Hillier SG (1991) Regulatory functions for inhibin and activin in human ovaries. J Endocrinol 131: 171-175.
- Nahum R, Thong KJ, Hillier SG (1995) Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod 10: 75-81.
- Shelling AN, Burton KA, Chand AL, van Ee CC, France JT, et al. (2000) Inhibin: a candidate gene for premature ovarian failure. Hum Reprod 15: 2644-2649.
- Fouany MR, Sharara FI (2013) Is there a role for DHEA supplementation in women with diminished ovarian reserve? J Assist Reprod Genet 30: 1239-1244.
- Ford JH (2013) Reduced quality and accelerated follicle loss with female reproductive aging-does decline in theca dehydroepiandrosterone (DHEA) underlie the problem? J Biomed Sci 20: 93.
- Diamanti-Kandarakis E, Kouli CR, Bergiele AT, Filandra FA, Tsianateli TC, et al. (1999) A survey of the polycystic ovary syndrome in the Greek island of Lesbos: hormonal and metabolic profile. J Clin Endocrinol Metab 84: 4006-4011.
- Franks S, Gilling-Smith C, Gharani N, McCarthy M (2000) Pathogenesis of polycystic ovary syndrome: evidence for a genetically determined disorder of ovarian androgen production. Hum Fertil (Camb) 3: 77-79.
- Nelson VL, Qin KN, Rosenfield RL, Wood JR, Penning TM, et al. (2001) The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J Clin Endocrinol Metab 86: 5925-5933.
- Yildiz BO, Azziz R (2007) The adrenal and polycystic ovary syndrome. Rev Endocr Metab Disord 8: 331-342.
- Dunaif A, Fauser BC (2013) Renaming PCOS--a two-state solution. J Clin Endocrinol Metab 98: 4325-4328.
- Welt CK, Carmina E (2013) Clinical review: Lifecycle of polycystic ovary syndrome (PCOS): from in utero to menopause. J Clin Endocrinol Metab 98: 4629-4638.
- Harlow BL, Signorello LB (2000) Factors associated with early menopause. Maturitas 35: 3-9.
- Fern M, Rose DP, Fern EB (1978) Effect of oral contraceptives on plasma androgenic steroids and their precursors. Obstet Gynecol 51: 541-544.
- Bellati F, Ruscito I, Gasparri ML, Antonilli M, Pernice M, et al. (2014) Effects of unilateral ovariectomy on female fertility outcome. Arch Gynecol Obstet 290: 349-353.
- Brook JD, Gosden RG, Chandley AC (1984) Maternal ageing and aneuploid embryos-evidence from the mouse that biological and not chronological age is the important influence. Hum Genet 66: 41-45.
- Augood C, Duckitt K, Templeton AA (1998) Smoking and female infertility: a systematic review and meta-analysis. Hum Reprod 13: 1532-1539.
- Tiboni GM, Bucciarelli T, Giampietro F, Sulpizio M, Di Ilio C (2004) Influence of cigarette smoking on vitamin E, vitamin A, beta-carotene and lycopene concentrations in human pre-ovulatory follicular fluid. Int J Immunopathol Pharmacol 17: 389-393.
- Kline J, Kinney A, Levin B, Warburton D (2000) Trisomic pregnancy and earlier age at menopause. Am J Hum Genet 67: 395-404.
- Tziomalos K, Charsoulis F (2004) Endocrine effects of tobacco smoking. Clin Endocrinol (Oxf) 61: 664-674.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences