Responses to the Bioactive Component of Crassocephalum crepidioides on Histomorphology, Spermatogenesis and Steroidogenesis in Streptozotocin-Induced Diabetic Male Rats
Adelakun SA and Ogunlade B
Adelakun SA* and Ogunlade B
Department of Human Anatomy, School of Health and Health Technology, Federal University of Technology, Akure, Ondo State, Nigeria
- *Corresponding Author:
- Adelakun SA
Department of Human Anatomy, School
of Health and Health Technology,
Federal University of Technology, PMB 704,
Akure, Ondo State, Nigeria
Tel: +2348133354835
E-mail: saadelakun@futa.edu.ng
Received Date: July 03, 2018; Accepted Date: August 27, 2018; Published Date: September 04, 2018
Citation: Adelakun SA, Ogunlade B (2018) Responses to the Bioactive Component of Crassocephalum crepidioides on Histomorphology, Spermatogenesis and Steroidogenesis in Streptozotocin- Induced Diabetic Male Rats. J Reproductive Endocrinol & Infert. Vol.3 No.1:6
Abstract
Male reproductive function is clearly impaired in diabetes. This study investigates the responses to the bioactive component of Crassocephalum crepidioides (C. crepidioides) on histomorphology, spermatogenesis and steroidogenesis in Streptozotocin induced Diabetic Male Rats. Eighteen (18) male diabetic and twelve non diabetic male rats were divided into five groups of six (n=6) rats each. Group A served as non diabetic (ND) control were given 2 mL/kg distilled water each twice daily, group B: Diabetic control (DC); diabetic rats given 2 mL/kg distilled water each daily as placebo, group C: 500 mgkg-1 body weight of C. crepidioides was administered orally twice daily in Non-Diabetic groups, group D:500 mgkg-1 body weight of C. crepidioides was administered orally twice daily in Diabetic groups; group E: Diabetic rats treated subcutaneously with 5 units/kg.bwt of insulin. The duration of the study was 28 days. Parameters tested include: fasting blood and serum glucose, sperm parameters, Testosterone (TT), Follicle stimulating hormone (FSH) and Luteinizing hormone (LH) and testicular histology. C. crepidioides significantly decreased blood and serum glucose levels and significantly improved sperm quality and TT, FSH and LH in diabetic rats when compared with the Non-diabetic control. Degeneration and inflammatory responses in the testicular cells of the diabetic rats were significant improved after the administration of the plant extracts. The present study suggested that C. crepidioides extract improved the quality, viability, motility of semen analysis and reproductive hormone thereby preserving the testicular functions and male genital organs in diabetic rats.
Keywords
Crassocephalum crepidioides; Spermatogenesis; Steroidogenesis; Streptozotocin; Diabetes; histomorphology
Introduction
Diabetes mellitus is one of the most common life threatening disorders of metabolism [1] and is globally regarded important public health problems [2]. Most male individual usually suffer from sub-fertility or infertility due to diabetic complication [3], through multifactorial mechanisms [4]. Several structural and functional alterations occurs in many organs due to diabetes such as testis, pancreas, and brain [5-7]. Diabetes complications has been reported to impair male reproductive functions both humans and animals studies [8,9]. These complications also impairs spermatogenesis and reduces sperm count, sperm motility, seminal fluid volume, and testosterone levels [6,8,9]. In addition, diabetics reduced the diameter of the seminiferous tubule and thickening of the basement membrane within the seminiferous tubules and degeneration of the germ cells in several diabetic animals [6]. C. crepidioides is known to contain a large number of phytochemical compounds which include: Alkaloids, Glycosides, Cardiac glycosides, Steroids, Coumarin, Tannins, Flavonoids, Saponins, Reducing sugar [10].
Infertility has been regarded as one of the major public health problems globally, and approximately 30 % of infertilities are due to a male factor [11,12]. Several diabetic complications interfere with spermatogenesis and reduce sperm quality and production such as drug treatment, chemotherapy, toxins, air pollutions and insufficient vitamins intake have harmful effects on spermatogenesis and sperm normal production [13]. Antioxidants and vitamin A, B, C, and E in diet can protect sperm DNA from scavengers such as free radicals and improve the mechanism of blood-testes barrier [14].
Crassocephalum crepidioides is also referred to as red flower ragleaf and also called ‘Ebolo’ (in Yoruba land in Nigeria). It is an annual edible plant that is found in several tropical and subtropical areas [15]. The fleshy C. crepidioides leaves and stems are usually eaten and have several medicinal importances [16]. Several studies on C. crepidioides revealed its significance in the treatment of indigestion, stomach ache, epilepsy, sleeping sickness, swollen lips and it has also antitumor activity associated with nitric oxide production [17]. In addition, it has antioxidant properties that protects against hepatotoxicity [18]. Furthermore, methanolic extract of C. crepidioides possesses promising anti-hyperlipidemic activity [19]. Therefore, the present study was designed to evaluate the responses to the bioactive component of Crassocephalum crepidioides on histomorphology, spermatogenesis and steroidogenesis in Streptozotocin- Induced Diabetic male rats.
Materials and Methods
Chemicals and reagents
Chemicals and reagents used in this study were of analytical grade and obtained from Sigma Chemical Company, St. Louis, Missouri, USA. Testosterone, Follicle stimulating hormone (FSH) and Luteinizing hormone (LH) were obtained from Randox Laboratories Ltd, Admore Diamond Road, Crumlin, Co., Antrim, United Kingdom.
Collection of Crassocephalum crepidioides
Crassocephalum crepidioides were harvested from the Research Farm, School of Agricultural Sciences and Technology, Federal University of Technology, Akure, Nigeria in April, 2018. The plants were collected in line with the guidelines for Good Practice Plant Collection proposed by the World Health Organization [20]. Samples of C. crepidioides were identified and authenticated by Dr. Ileke Department of Biology, Federal University of Technology, Akure, Nigeria. A voucher of Ccrepidioides specimen deposited for reference purpose.
Preparation of the Crassocephalum crepidioides extract
The collected samples of C. crepidioides were air dried at room temperature and powdered with the aids of electronic blender thermo cool (model TC234H, China) and subjected to extraction. Five hundred grams of the powder was mix with about 2500 mL of 80% methanol for 15 days. The extract was filtered and mixes twice with same volume of methanol to extract C. crepidioides [21]. The methanol was evaporated from the extract under reduced pressure by rotary evaporator (Rotavapor® R-211) at 45°C. The resulting dry extract yield 23% (w/w). The dried C. crepidioides extract then mixed with distilled water for administration. The approximate analysis of the grinded leaves was evaluated by Official Methods of Analysis, Association of Official Agriculture Chemists (AOAC) [22].
Experimental animal
In this study adult male rats aged 12-14 weeks old, weighing 200 ± 20 g were used. The rats were obtained from the Nigerian Institute of Medical Research (NIMR), Yaba Lagos, Nigeria. The rats were housed in well ventilated plastic cages with 12 h light, dark cycle at 25 ± 2°C and 45-55% relative humidity in the Human Anatomy Department Animal Control Room. The rats were fed with dose of 100 g/kg standard rat chow as recommended and advised by the International Centre of Diarrheal Disease Research, Bangladesh (ICDDR, B) daily. The rats have access to water ad libitum. The animals were acclimatized for two weeks before commencement of the administration because they are sensitive to environmental changes. All experimental procedures followed the recommendations provided in the “Guide for the Care and Use of Laboratory Animals by National Institute of Health [23].
Induction of experimental diabetes
Diabetes induced by a single intraperitoneal injection of streptozotocin (STZ) solution (60 mg/kg body weight) in acidified saline solution and used within 10 min of preparation [24]. Control animals received only the acidified saline solution at pH 4.5. Diabetic rats were those with blood glucose greater than 250 mg/dL, 72 h after injection of streptozotocin [25] 10% sucrose was added to the water in the first 48 h after intraperitoneal injection of STZ solution to prevent hypoglycemia. Diabetes was confirmed by the drop of blood from the tail for glucose level estimation using Glucometer meter (ACCU-CHEK Active, Roche Diagnostics, Germany) and allowed for 2 weeks diabetic stabilization before being used for the experiment.
Experimental design
Thirty adult male wistar rats; Eighteen (18) male diabetic and Twelve (12) non diabetic rats were randomly assigned into five groups of six (n=6) rats each. Group A served as non diabetic (ND) control were given distilled water 2 mL/kg each twice daily for 28 days, group B: Diabetic control (DC); diabetic rats given 2 mL/ kg distilled water each daily as placebo, group C: Non Diabetic rats treated orally with 500 mg/kg body weight of C. crepidioides twice daily, group D: Diabetic rats treated orally with 500 mg/ kg body weight of C. crepidioides twice daily, group E: Diabetic rats treated subcutaneously with 5 units/kg.bwt of insulin. The experimental period was 28 days. At the end of the experimental period the rats were fasted overnight but had free access to water. The rats were euthanized using chloroform vapor and sacrificed. Immediately, blood samples were collected for sera preparation by cardiac puncture into sterile plain tubes. Serum samples separated from the clot by centrifugation for 10 minutes at 3,000 rpm using bench top centrifuge (MS23E Minor, England) and stored frozen until needed for analysis. All analysis was completed within 24 hours of sample collection.
Sperm analysis
Sperm cells were collected from the epididymes as reported Amelar et al. [26]. Briefly, testis was excised and caudal epididymis was isolated and placed in a Petri dish containing 3 mL of NaHCO3 buffered Tyrodes’s Lactate solution. Several (1 mm) incisions were made on it and sperm was gently drawn into a plastic transfer pipette and transferred into 5 mL test tubes and shake vigorously for homogeneity and dispersal of sperm cells. Sperm was therefore analyzed to determine sperm motility, sperm count, percentage of abnormal sperm cells (sperm morphology) and percentage of viable sperm cells (sperm viability) following standard procedures [27].
Hormone determination
Testosterone (TT), follicle stimulating hormone (FSH) and luteinizing hormone (LH) level in serum were measured using Enzyme-Linked Immunoasorbent Assay (ELISA) kit (Diagnostic automation Inc, CA) in line with manufacturer’s instructions.
Histopathological analysis
The testis were harvested and fixed in Bouin‘s fluid. The testicular tissues were embedded in paraffin and tissue sections (5 μm) stained with hematoxylin and eosin (H and E) and examined with light microscope (Nikon Eclipse EN400). Histopathological changes between control and experimental animals were noted. All alterations from the normal structure were registered. The images were photographed with an Olympus Model BS54 microscope at a magnification of 200x.
Data presentation
Data expressed as Mean ± SEM. Statistical differences between the groups were evaluated by one way ANOVA, followed by Newman-Keuls Multiple Comparison Test. Differences yielding p<0.05 considered statistically significant. Statistical analyses of data were performed using Graph Pad Prism 5 Windows (Graph Pad Software, San Diego, California, USA).
Results
Blood and serum glucose levels in diabetic and non-diabetic rats
There was elevated glucose blood level in DC group to 441.40 ± 31.62 mg/dL while its final concentration of 342.40 ± 18.73 mg/dL was significantly higher than CC (76.59 ± 5.32 mg/dL), D+CC (142.30 ± 13.45 mg/dL), D+INSULIN (82.57 ± 3.00 mg/ dL), and N. Control (74.61 ± 6.02 mg/dL) final concentrations. The serum glucose level in the DC group (12.70 ± 0.47 mmo/dl) was significantly (p<0.05) elevated when compared to N. Control (4.70 ± 0.47 mmol/L), while D+CC (8.12 ± 0.77 mmol/L) showed a rather significant decrease (p<0.05) in the serum glucose compared with the DC (12.55 ± 1.62 mmol/L) (Table 1).
Table 1. Responses to the bioactive component of Crassocephalum crepidioideson blood and serum glucose levels of diabetic and non-diabetic rats.
| Fasting blood glucose levels | |||
|---|---|---|---|
| Groups | Initial (mg/dl) | Final (mg/dl) | Serum glucose (mmol/L) |
| N.Control | 95.25 ± 14.16 | 74.61 ± 6.02 | 4.70 ± 0.47 |
| DC | 441.40 ± 31.62* | 342.40 ± 18.73* | 12.55 ± 1.62* |
| CC | 99.54 ± 17.71 # | 76.59 ± 5.32# | 4.17 ± 0.52*,# |
| D+CC | 469.70 ± 25.09* | 142.30 ± 13.45*,# | 8.12 ± 0.77*,# |
| D+INSULIN | 481 ± 90 ± 25.81* | 82.57 ± 3.00# | 4.27 ± 0.32*,# |
±: Mean of SEM;
*,#: were considered significant compared to Normal control and Diabetic control groups respectively.
Sperm motility, sperm concentration and sperm viability in diabetic and non-diabetic rats
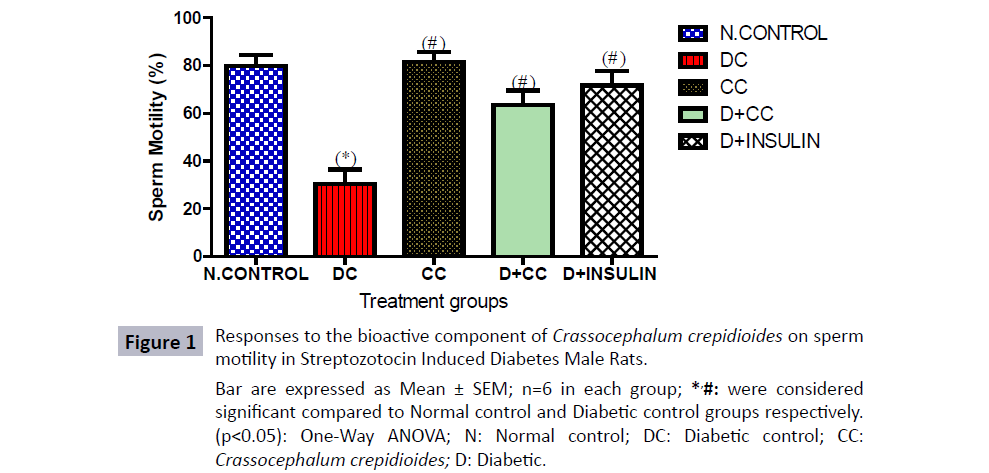
As shown in Figure 1, there was observed significant decrease in sperm motility of diabetic control groups that was given 2 mL/kg distilled water as placebo, diabetic group treated with 500 mg/ kg bwt of C. crepidioides and 5 units/kg.bwt of insulin compared to the non diabetic control group. Furthermore, there was a statistically significant increase (p<0.05) in the diabetic group administered with 500 mg/kg bwt of C. crepidioides and 5 units/ kg.bwt of insulin compared with diabetic control group. Also there was increase in mean value of C. crepidioides only treated group when compared with the positive control and model diabetic control group.
Figure 1: Responses to the bioactive component of Crassocephalum crepidioides on sperm
motility in Streptozotocin Induced Diabetes Male Rats.
Bar are expressed as Mean ± SEM; n=6 in each group; *,#: were considered
significant compared to Normal control and Diabetic control groups respectively.
(p<0.05): One-Way ANOVA; N: Normal control; DC: Diabetic control; CC: Crassocephalum crepidioides; D: Diabetic.
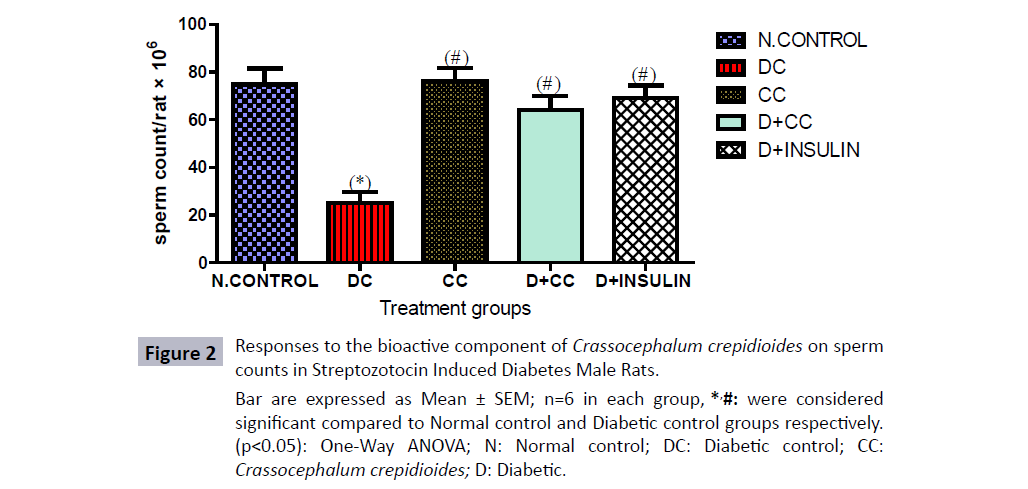
There was a significant decrease (p<0.05) in sperm concentration of diabetic control groups treated with 2 mL/kg distilled water as placebo, diabetic group treated with 500 mg/kg bwt of C. crepidioides and 5 units/kg.bwt of insulin compared with control group. However, a significant increase (p<0.05) was observed in diabetic group treated with 500 mg/kg bwt of C. crepidioides and 5 units/kg.bwt of insulin when compared with diabetic control group. Also there was increase mean value of C. crepidioides only treated group in comparison with normal control and significant increase (p<0.05) was evident compared with the diabetic control group (Figures 2-4).
Figure 2: Responses to the bioactive component of Crassocephalum crepidioides on sperm
counts in Streptozotocin Induced Diabetes Male Rats.
Bar are expressed as Mean ± SEM; n=6 in each group, *,#: were considered
significant compared to Normal control and Diabetic control groups respectively.
(p<0.05): One-Way ANOVA; N: Normal control; DC: Diabetic control; CC: Crassocephalum crepidioides; D: Diabetic.
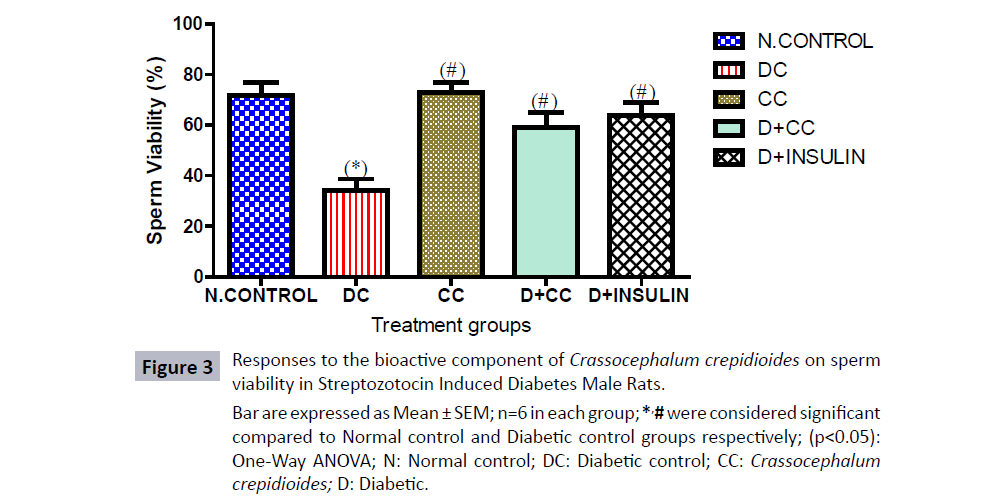
Figure 3: Responses to the bioactive component of Crassocephalum crepidioides on sperm
viability in Streptozotocin Induced Diabetes Male Rats.
Bar are expressed as Mean ± SEM; n=6 in each group; *,# were considered significant
compared to Normal control and Diabetic control groups respectively; (p<0.05):
One-Way ANOVA; N: Normal control; DC: Diabetic control; CC: Crassocephalum
crepidioides; D: Diabetic.
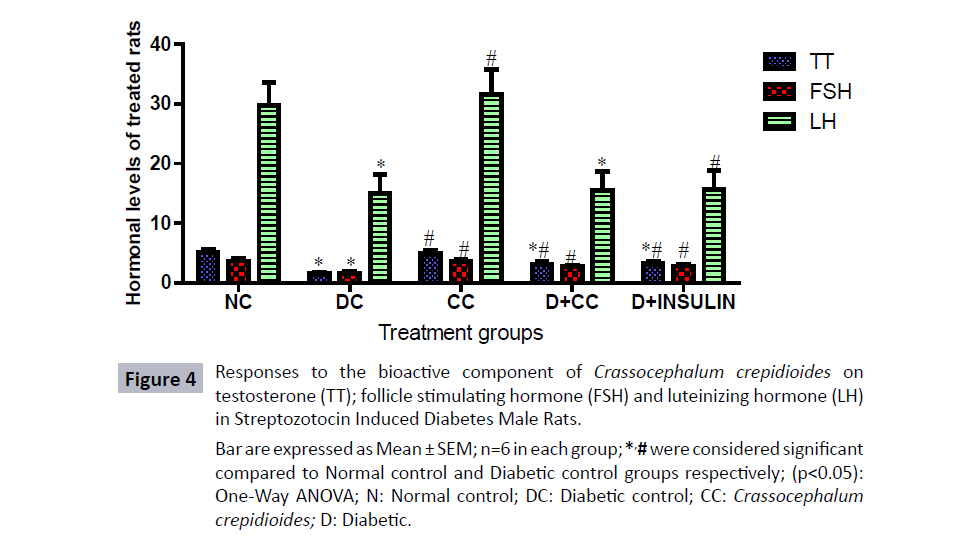
Figure 4: Responses to the bioactive component of Crassocephalum crepidioides on
testosterone (TT); follicle stimulating hormone (FSH) and luteinizing hormone (LH)
in Streptozotocin Induced Diabetes Male Rats.
Bar are expressed as Mean ± SEM; n=6 in each group; *,# were considered significant
compared to Normal control and Diabetic control groups respectively; (p<0.05):
One-Way ANOVA; N: Normal control; DC: Diabetic control; CC: Crassocephalum
crepidioides; D: Diabetic.
The sperm viability showed a significant decrease (p<0.05) in sperm viability in the diabetic control groups administered with 2 mL/kg distilled water as placebo when compared with the control group, there was also a significant decrease (p<0.05) in sperm viability of diabetic group treated with 500 mg/kg bwt of C. crepidioides and 5 units/kg.bwt of insulin when compared with the normal control. However, sperm viability was significantly improved (p<0.05) in diabetic group treated with 500 mg/kg bwt of C. crepidioides and 5 units/kg.bwt of insulin when compared with the diabetic control group treated with 2 mL/kg distilled water as placebo. There was statistical significant increased (p<0.05) in sperm viability within the group treated with 500 mg/ kg bwt of C. crepidioides only compared to the diabetic control group.
Hormonal assay in diabetic and non diabetic groups
The level of serum testosterone (TT), Follicle stimulating hormone (FSH) and LH concentrations was significantly increased (p<0.05) in diabetic rats treated with either 500 mg/kg bwt of C. crepidioides or 5 units/kg.bwt of the insulin level compared to the diabetic control group treated with 2 mL/kg distilled water as placebo (Figure 4). In addition, the level of TT, FSH and LH concentration was significantly reduced (p<0.05) in the diabetic control group compared to the normal control group. Furthermore, there was significant increase (p<0.05) in TT, FSH, and LH level in group treated with 500 mg/kg bwt of C. crepidioides only when compared with diabetic control group.
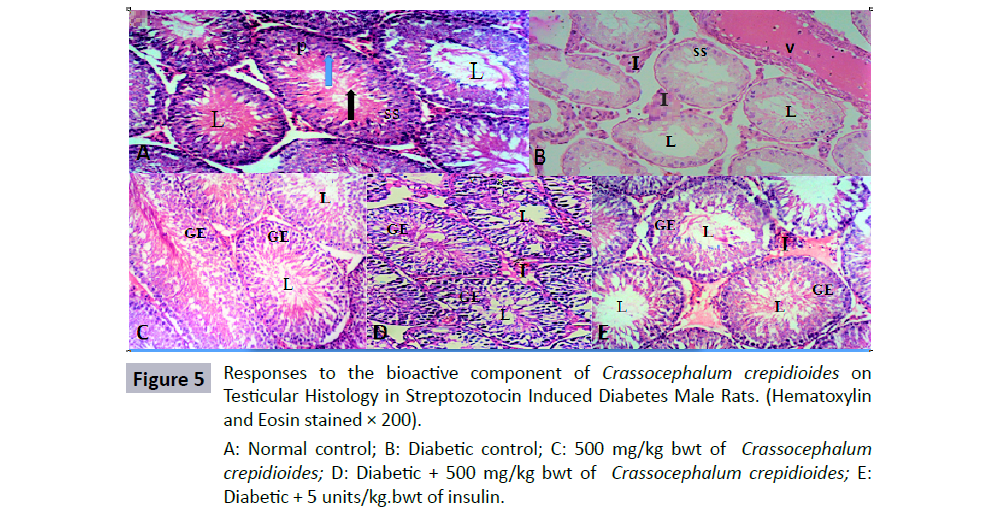
Testicular histology in diabetic and non-diabetic rats
In Figure 5, the photomicrograph of the seminiferous tubules of the control group was moderately circular or ovoid in appearance showing normal stratified epithelium with numerous spermatogonic cells and spermatozoa within the lumen. Composed of 2 major cells, which are the supporting cells (sertoli cells) and spermatogenic cells. The spermatozoa are arranged in rows between and around the cells of sertoli. The seminiferous tubules of the diabetic rats treated with distil water alone as placebo revealed severe decrease in the spermatogenic cells, reduced cellularity of the interstiumm, lumen widening, tubular atrophy and reduction in spermatozoa within the lumen. There were matured spermatozoa in the seminiferous tubules of non diabetic rats treated with C. crepidioides only. The cytoarchitecture of the diabetic rats of C. crepidioides and group treated with insulin showed normal cellular composition in the germinal epithelium with numerous sperm cells within the lumen and normal interstitium.
Figure 5: Responses to the bioactive component of Crassocephalum crepidioides on
Testicular Histology in Streptozotocin Induced Diabetes Male Rats. (Hematoxylin
and Eosin stained × 200).
A: Normal control; B: Diabetic control; C: 500 mg/kg bwt of Crassocephalum
crepidioides; D: Diabetic + 500 mg/kg bwt of Crassocephalum crepidioides; E:
Diabetic + 5 units/kg.bwt of insulin.
Values are expressed as Mean ± SEM, n=6 in each group, *,# were considered significant compared to Normal control and Diabetic control groups respectively. (p<0.05), One-Way ANOVA. N: Normal control, DC: Diabetic control, CC: Crassocephalum crepidioides, D: Diabetic (A) Micrograph of testis of control rats, seminiferous tubules shows cells of the spermatogenic series (SS) and lumen (L); blue arrow represents spermatogonium; P represents primary spermatocytes; black arrow represents spermatids and spermatozoa. (B) Micrograph of testis of diabetic rat showing hypocellularity, few cells of spermatogenic series (SS), shortening and sloughing of germinal epithelium, widened empty lumen (L); widened interstitium (I) and vascular haemorrage (V). (C-E) Micrograph of testis showing normal germinal epithelium (GE) with sperm cells in the lumen (L).
Discussion
Diabetes-induced testicular dysfunction might be transient depending on the duration and degree of the disease and erectile problem is a well-recognized complexity of diabetes mellitus [28].
In this study, streptozocin-induced hyperglycaemia was deduced to be a useful experimental model in studying the activity of antidiabetic agents [29,30]. Pancreatic insulin secreting β cells selectively destroyed by Streptozocin, leaving less active cell resulting in a diabetic state [31]; which was the observation in diabetics control of this study.
Current study indicates significant increase in the blood glucose levels in diabetic animals and was drastically reduced after treatment with C. crepidioides. This suggests hypoglycemic activities and anti-diabetic effect of the plant. Daily treatment with the extract of C. crepidioides led to a fall in blood and serum sugar levels, with the effect reaching maximum after 15 days of treatment and remains constant up to 28 days; this conformed with the report of Rao and Naidu [32]. In diabetic control group serum glucose levels was significantly increased but interventions of C. crepidioides extract ameliorate this effect. Reductions in blood and serum glucose in the treated diabetic rats observed concur with the reports of Mustafa et al. [33] and Olanrewaju et al. [34]. The plant is known to contain a large number of phytochemical compounds which include: Phytate, Tannin, Saponin, Oxalate, HCN and good sources of vegetables nutrients especially vitamin A, C, D, E, Folate and mineral Na, Ca, K, Mg, P [10,35] may be responsible for this observation and the possible mechanism though not investigated may be due to the ability of the extract to potentiate insulin secretion from pancreatic beta cells or sensitizing insulin receptors [36].
Sperm characterization is a vital property of sperm and male fecundity decreases progressively with reduction in sperm concentrations [37]. However, sperm motility is a critical indicator of semen quality and fertility potential [27,38].
Our observations from this study showed that diabetes could induce male infertility as revealed in the semen analysis. This is due to hyperglycaemia which is seen in diabetes was as a result of inability of glucose to enter the testes cells affected the Leydig cells and cells of Sertoli which are to produce testosterone and sperm cells through spermatogenesis, this result to under secretion of testosterone in diabetic rats which led to reduction in the rate of spermatogenesis and finally the sperm count, motility and viability. These findings concur with previous report that diabetes could indeed result to infertility in male rats [39,40].
Hyperglycemia of diabetes mellitus raises the level of free radicals especially the reactive oxygen species (ROS) that produces DNA destruction in testis and a major decrease in sperm parameters which includes sperm motility, count, and viability [41-45]. However administration of C. crepidioides improves the sperm parameters by scavenging the reactive oxygen species and activates testicular enzymatic antioxidant status. The sperm cell count is the most critical assessment value for spermatogenesis, and it is highly associated with fertility. In this study, diabetic rats showed a marked decrease in sperm concentration, motile sperm percentage, and increased sperm abnormalities. This is constant with the report of Scarano et al. [41] and Mustafa et al. [32] reported that there was reduction in sperm quantity and quality in diabetic rats as a result of association of oxidative injuries. Also diabetes induced oxidative stress has been reported to cause peroxidation of sperm membrane lipid which might interfere with membrane fluidity and transport processes [43]. In view of this, appearance of various abnormal sperm shapes could be due to abnormal membrane or cellular and nuclear changes induced by diabetes [44].
High testosterone level is essential for the normal physiology of seminiferous tubules and testicular testosterone is crucial for spermatogenesis [45], in our study, testosterone decreased in diabetic rats, as also found by Farrell et al. [45] and it significantly improved in the Diabetic+CC group. This finding explores the protective effect of C. crepidioides on spermatogenesis. Ballester et al. [46] suggested that the low level of testosterone in diabetic rats may be related to the decrease in Leydig cells or in androgen biosynthesis. Diabetic rats showed decreased testosterone, follicle stimulating hormone (FSH), and luteinizing hormone (LH) level in the current study; this finding is in harmony with results from Ballester et al. [46] reported that diabetic rats displayed a reduced concentration in serum insulin, testosterone, follicle stimulating hormone (FSH), and luteinizing hormone (LH) [47]. Interventions of C. crepidioides therefore elevate the hormone level in diabetic rats (Figures 4 and 5).
In this study, there was decreased cellularity in the spermatogenic series due to degeneration, sloughing and shortening of seminiferous epithelium in diabetic rats. This is constant with previous study by Arash et al. [47] who reported that diabetes significantly inhibited the proliferative activity of the spermatogonia in all stages of the seminiferous tubules cycle and destroyed intra tubular space, atrophied seminiferous tubules with degeneration, intra tubular space fibrosis and inflammatory cells (lymphocyte) in diabetic rats. C. crepidioides extract maintained the histomorphology of the testis by increasing the proliferative activity of spermatogonia compared to the control animals. From our observation, when C. crepidioides extract was administered to diabetic rats; it’s thereby protect the testis from the pernicious effects of diabetes. The protective nature of C. crepidioides could be attributed to various phytochemical constituents such as the presence of ascorbic acid which is known for its protection on cell membranes and its scavenging effects on free radicals. This study therefore confirmed administration of C. crepidioides extract ameliorates reproductive dysfunctions in diabetic rats [48].
Conclusion
This suggested that Crassocephalum crepidioides extract has significant beneficial effects on the sperm count, viability, motility, reproductive hormone and histomorphology of the testis and could be effective for maintaining healthy sperm parameters and male reproductive function in diabetic rats.
References
- Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030”. Diabetes Res Clin Pract 87: 4-14.
- Wild S, Roglic G, Green A, Sicree R, King H (2004) Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 27: 1047-1053.
- La Vignera S, Condorelli R, Vicari E, D’Agata R, Calogero AE (2012) Diabetes mellitus and sperm parameters. J Androl 33: 145-153.
- Shrilatha B, Muralidhara (2007) Early oxidative stress in testis and epididymal sperm in streptozotocin-induced diabetic mice: Its progression and genotoxic consequences. Reprod Toxicol 23: 578-587.
- Aksu I, Baykara B, Kiray M, Gurpinar T, Sisman AR, Ekerbicer N, et al. (2013) Serum IGF-1 levels correlate negatively to liver damage in diabetic rats. Biotech Histochem 88: 194-201.
- Guneli E, Tugyan K, Ozturk H, Gumustekin M, Cilaker S, et al. (2008) Effect of melatonin on testicular damage in streptozotocin-induced diabetes rats. Eur Surg Res 40: 354-360.
- Uysal N, Yalaz G, Acikgoz O, Gonenc S, Kayatekin BM (2005) Effect of L-carnitine on diabetogenic action of streptozotocin in rats. Neuro Endocrinol Lett 26: 419-422.
- Oksanen A (1975) Testicular lesions of streptozotocin diabetic rats. Horm Res 6: 138-144.
- Sexton WJ, Jarow JP (1997) Effect of diabetes mellitus upon male reproductive function. Urology 49: 508-513.
- Entaz B, Maknoon SS, Bashutosh N, Hyonok Y (2016) Evaluation of In vitro Antioxidant and In vivo Antihyperlipidemic Activities of Methanol Extract of AerialPart of Crassocephalum crepidioides(Asteraceae) Benth S Moore. Trop J Pharm Res 15: 481-488.
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE (1992) Evidence for decreasing quality of semen during past 50 years. BMJ 305: 609-613.
- Isidori AM, Pozza C, Gianfrilli D, Isidori A (2006) Medical treatment to improve sperm quality. J Reprod Biomed Online 12: 704-714.
- Mosher WD, Pratt WF (1991) Fecundity and infertility in the United States: incidence and trends. J Fertil Steril 56: 192-193.
- Khaki A, Fathiazad F, Nouri M, Khaki AA, Ghanbari Z, et al. (2011) Anti-oxidative effects of citro flavonoids on spermatogenesis in rat. Afr J Pharm Pharmacol 5: 721-725.
- Musa AA, Adekomi DA, Tijani AA, Muhammed OA (2011) Some of the Effect of Crassocephalum crepidioideson the Frontal cortex, Kidney, Liver and Testis of Adult Male Sprague Dawley Rats: Microanatomical Study. Eur J Exp Biol 1: 228-235.
- Grubben GJH (2004) Plant Resources of Tropical Africa: Vegetables. PROTA Foundation Leiden: Backhuys Publishers 67-72.
- Tomimori K, Nakama S, Kimura R, Tamaki K, Ishikawa C (2012) Antitumor activity and macrophage nitric oxide producing action of medicinal herb, Crassocephalum crepidioides. BMC Complement Altern Med 12: 78.
- Aniya Y, Koyama T, Miyagi C, Miyahira M, Inomata C, et al. (2005) Free radical scavenging and hepatoprotective actions of the medicinal herb, Crassocephalum crepidioides from the Okinawa Islands. Biol Pharm Bull 28: 19-23.
- Bahar E, Siddika MS, Nath B, Yoon H (2016) Evaluation of In vitro Antioxidant and In vivo Antihyperlipidemic Activities of Methanol Extract of Aerial Part of Crassocephalum crepidioides (Asteraceae) Benth S Moore. Trop J Pharm Res 15: 481-488.
- WHO (1999) WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th Edn Cambridge University Press, New York, USA. 128.
- Hailu W, Engidawork E (2014) Evaluation of the diuretic activity of the aqueous and 80% methanol extracts of Ajuga remota Benth (Lamiaceae) leaves in mice. BMC Complement Altern Med 14: 135.
- AOAC (1975) Official Methods of Analysis. Association of Official Agriculture Chemists, Washington, DC. 811-817.
- NIH (1985) National Institutes of Health Guide for the Care and Use of Laboratory Animals: DHEW Publication (NIH), revised. Office of Science and Health Reports, DRR/NIH, Bethesda, USA.
- Tunali S, Yanardag R (2013) Protective effect of vanadyl sulfate on skin injury in streptozotocin-induced diabetic rats. Hum Exp Toxicol 32: 1206-1212.
- Burcelin R, Eddouks M, Maury J, Kande J, Assan R, Girard J (1995) Excessive glucose production, rather than insulin resistance, accounts for hyperglycaemia in recent-onset Streptozotocin diabetic rats. Diabetologia 38: 283-290.
- Amelar RD, Dubin L, Schoenfeld C (1973) Semen analysis: An office technique. Urology 2: 606-611.
- Altay B, Cetinkalp S, Doganavsargil B, Hekimgil M, Semerci B (2003) Streptozotocin-induced diabetic effects on spermatogenesis with proliferative cell nuclear antigen immunostaining of adult rat testis. Fertil Steril 80: 828-831.
- Szkudelski T (2001) The mechanism of alloxan and streptozotocin action in β cells of the rat pancreas. Physiol Res 50: 537-546.
- Arikawe AP, Oyerinde A, Olatunji-Bello, Obika LO (2012) Streptozotocin Diabetes and Insulin Resistance Impairment of Spermatogenesis in Adult Rat Testis: Central Vs local mechanism. Niger J Physiol Sci 27: 171-179.
- Moore MC, Coate KC, Winnick JJ, Zhibo AN, Cherrington AD (2012) Regulation of hepatic glucose uptake and storage In vivo. In thematic review series: Nutrient Control of Metabolism and Cell Signaling American Society for Nutrition. Adv Nutr 3: 286-294.
- Rao PV, Naidu MD (2010) Anti-diabetic effect of Rhinacanthus nasutus leaf extract in streptozotocin induced diabetic rats. Libyan Agric Res Center J Int 1: 310-312.
- Mustafa SA, Essam AA, Ali HE, Rasha MS, Doaa HA, et al. (2017) Thymoquinone Defeats Diabetes-Induced Testicular Damage in Rats Targeting Antioxidant, Inflammatory and Aromatase Expression. Int J Mol Sci 919: 1-15.
- Olanrewaju AJ, Olatunji SY, Owolabi JO, Oluwatosin AT, Amaechi WC (2017) Invivo Evidences of Curcuma Longa on oxidative stress in STZ-induced diabetes on sperm parameters in male wistar rats. Anat J Afr 6: 1045-1051.
- Nupo SS, Onigbogi IO, Akinlotan JV, Ilori OA (2013) Effect of different processing methods on the nutrients and antinutrient composition of senecio biafrae, crassocephalum crepidiodes and solanum nigrum consumed in south west Nigeria. Am J Food Nutr 3: 147-154.
- Budin SB, Yusof KM, Idris MHM, Hamid ZA, Mohamed J (2011) Tocotrienol-rich fraction of palm oil reduced pancreatic damage and oxidative stress in streptozotocin-induced diabetic rats. Aust J Basic Appl Sci 5: 2367-2374.
- Maya WC (2010) Sperm count. Do we need a new reference value? Arch Esp Urol 63: 133-138.
- Zinaman MJ, Brown CC, Selevan SG, Clegg ED (2000) Semen quality and human fertility: A prospective study with healthy couples. J Androl 21: 145-153.
- Dockery F, Bulpit CJ, Agarwal S, Donaldson M, Rajkumar C (2003) Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 104: 195-201.
- Nishiyama T, Ishizaki F, Anraku T, Shimura H, Takahashi K (2005) The influence of androgen deprivation therapy on metabolism in patients with prostate cancer. J Clin Endocrinol Metab 90: 657-660.
- Amaral S, Oliveira PJ, Ramalho-Santos J (2018) Diabetes and the impairment of reproductive function: Possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev 4: 46-54.
- Scarano WR, Messias AG, Oliva SU, Klinefelter GR, Kempinas WG (2006) Sexual behaviour, sperm quantity and quality after short-term streptozotocin induced hyperglycaemia in rats. Int J Androl 29: 482-488.
- Sanocka D, Kurpisz M (2004) Reactive oxygen species and sperm cells. Reprod Biol Endocrinol 2: 12.
- Suresh S, Prithiviraj E, Venkata Lakshmi N, Karthik Ganesh M, Ganesh L (2013) Effect of Mucuna pruriens (Linn.) on mitochondrial dysfunction and DNA damage in epididymal sperm of streptozotocin induced diabetic rat. J Ethnopharmacol 145: 32-41.
- Sharpe RM, Kerr JB, McKinnell C, Millar M (1994) Temporal relationship between androgen-dependent changes in the volume of seminiferous tubule fluid, lumen size and seminiferous tubule protein secretion in rats. J Reprod Fertil 101: 193-198.
- Farrell JB, Deshmukh A, Baghaie AA (2008) Low testosterone and the association with type 2 diabetes. Diabetes Educ 34: 799-806.
- Ballester J, Munoz MC, Dominguez J, Rigau T, Guinovart JJ (2004) Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl 25: 706-719.
- Arash K, Fatemeh F, Mohammad N, AmirAfshin K, Navid AM (2010) Beneficial Effects of Quercetin on Sperm Parameters in Streptozotocin-Induced Diabetic Male Rats.Phytother Res.
- Eskenazi B, Kidd SA, Marks AR, Sloter E, Block G, Wyrobek AJ (2005) Antioxidant intake is associated with semen quality in healthy men. Hum Reprod 20: 1006-1012.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences