Assessment of Quality of Life and Therapeutic Outcomes of Metformin, Pioglitazone with Myoinositol and Ethinylestradiol Cyproterone in Ethnic Pakistani Women with Polycystic Ovary Syndrome
Khan A*
DOI10.36648/2476-2008.2021.6.11
Khan A* and Shahid A
Department of Pharmacy, Quaid-I-Azam University, Islamabad, Pakistan
- *Corresponding Author:
- Khan A
Department of Pharmacy
Quaid-i-Azam University
Islamabad, Pakistan
Tel: +92-51 9064-4181
E-mail: akhan@qau.edu.pk
Received date: October 05, 2018; Accepted date: February 07, 2019; Published date: February 14, 2019
Citation: Khan A, Shahid A (2019) Assessment of Quality of Life and Therapeutic Outcomes of Metformin, Pioglitazone with Myoinositol and Ethinylestradiol Cyproterone in Ethnic Pakistani Women with Polycystic Ovary Syndrome. J Rep Endo Infert. Vol.4 No.1:11
Keywords
Polycystic ovary syndrome; Quality of life; Pakistani; Metformin; Pioglitazone; Ethinylestradiol cyproterone
Abstract
Background: Only a few studies are available on improvement in quality of life after reviewing treatment in Polycystic Ovary Syndrome (PCOS) patients and no study was found in ethnic Pakistani women. This study was aimed to compare the outcomes of metformin, pioglitazone and ethinylestradiol cyproterone as changes in Quality of Life (QoL), clinical features, and adverse drug reactions, in patients suffering from PCOS.
Methods: This observational study was carried out at 4 public and private sector infertility centers in Islamabad. Patients aged 18-43 yrs, as per Rotterdam criteria diagnosed as PCOS were included in the study. Chi-PCOSQ as a measure of HRQoL. Patients were stratified according to BMI, infertility and family history of diabetes mellitus to further analyze features of these groups. Paired-t-test and regression analysis was applied to study changes in patient’s quality of life after 3 months of treatments given. Findings: We recruited 160 patients (20.6% obese, 61.2% infertile, and 46.2% patient’s presents with family diabetes history). Mean score of Chi-PCOSQ was 98.4 ± 24.2 with lowest in infertility (10.3 ± 7.9) domain. Metformin improved hair loss (p=0.015), pelvic pain (0.007), oligo menorrhea (p=0.001) and in Chi-PCOSQ depression domain score (p=0.002). Whereas pioglitazone improved acne (p=0.01), oligo menorrhea (p=0.031) and depression domain score (p=0.013). Ethinylestradiol cyproterone improved acne (p=0.003), menorrhagia (p=0.01) symptoms and in Chi-PCOSQ menstrual, acne and hair loss domain score (p=0.004 & p=0.001, respectively). A few of the patients also reported adverse drug reactions e.g. Ethinylestraiol cyproterone and metformin resulted in gastrointestinal upset in 21.4% and 11.5% patients, respectively.
Conclusion: Given drugs improved patients, clinical features and HRQoL while no drug significantly improved patient’s hirsutism related problems. PCOS adversely affects patient’s QOL and there was variation in clinical features from patient to patient. So, PCOS must be treated with drugs specifically considering their clinical features.
Abbreviations:
Chi-PCOSQ: Chinese version of health-related Quality-of-Life Questionnaire for women with Polycystic Ovary Syndrome; HRQoL: Health Related Quality of Life; PCOS: Polycystic Ovary Syndrome; PCOSQ: Health-related Quality-of-Life Questionnaire for women with Polycystic Ovary.
Background
According to World Health Organization (WHO) health is defined as, “A state of complete physical, mental, and social well-being not merely the absence of disease" [1]. Health cannot be measured only on the basis of changes in severity and frequency of disease symptoms and number of life savings it must be defined using properly designed tools i.e. Health Related Quality of Life (HRQoL) [2]. HRQoL in general point of view is an individual's or a group's perceived physical and mental health over time. Therefore nowadays, improvement in patient’s quality of life is ultimate goal of healthcare professionals [3]. Specifically, in the case of chronic diseases HRQoL can give us best idea about patient satisfaction and health status. It is also a best measure when we have to compare various treatment plans having similar impacts on patients life expectancy [4,5]. This study was aimed to compare the outcomes of metformin, pioglitazone with myoinositol and ethinylestradiol cyproterone, in terms of changes in clinical features and HRQoL of PCOS patients with different drugs. As stated by U.S.FDA guidance for industry, HRQoL is a measure to assess outcome of treatment. By using disease specific HRQoL tools we can estimate extent of improvement in disease symptoms [4].
One of most common endocrine-metabolic disorders in reproductive aged women is Polycystic Ovary Syndrome (PCOS). PCOS being a syndrome is frustrating and complex disorder [6,7]. Its prevalence is up to 26% worldwide, which varies with ethnicity and genetics in presentation of clinical features [7-10]. Mainly, PCOS can be defined as imbalance of hormones [10]. Firstly, in 1935 Stein and Leventhal reported a link between polycystic ovaries, oligomenorrhea and hirsutism [11]. After that different diagnostic criteria’s reported including NIH/NICHD (National Institute of Health/National Institute of Child Health and Human Development), Rotterdam criteria and androgen excess society criteria [12,13]. These criteria’s vary in phenotypes i.e. 1) Hyperandrogenism+oligoanovulation+ polycystic ovaries; 2) Hyperandrogenism+oligoanovulation; 3) Hyperandrogenism+polycystic ovaries; and 4) Oligo-anovulation+polycystic ovaries without hyperandrogenism. However, in 2012 NIH recommended Rotterdam criteria as it includes all the phenotypes [14]. PCOS patients presents with different characteristics which can be, metabolic (obesity, insulin resistance, and diabetes), endocrinological (oligo/amenorrhea, hirsutism, acne, hair loss, infertility and darkened skin) and emotional derangements [12-14]. It is also associated with comorbidities like diabetes mellitus, heart diseases, epilepsy, dyslipidemia etc. [14]. Treatment of PCOS depends on clinical features and complexity of disease. It starts from lifestyle modification (e.g. Atkins diet) to drugs (for example, clomiphene citrate, insulin sensitizing agents, oral contraceptives) and surgical procedures (laparoscopic drilling) depending upon the characteristics presented at the time of diagnosis [15-17].
Methodology
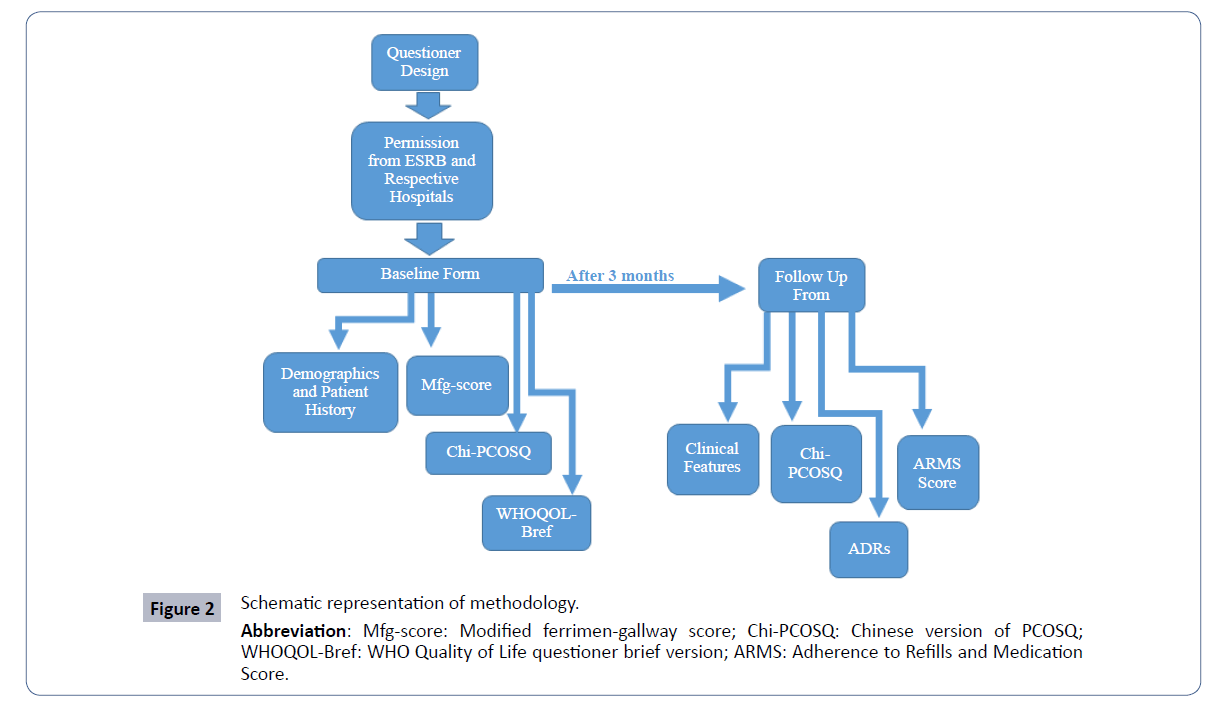
It is a prospective, observational study. Study permission was taken from ethical and scientific research board of Quaid-i- Azam University, Islamabad (BEC-FBS-QAU-111) and respective hospitals and infertility clinics. Schematic representation of methodology (Figures 1 and 2).
Participants
We recruited 160 PCOS patients out of which we got follow-up of only 120 patients. The reason of not getting follow-up was patients started IVF/ICSI (In-vitro Fertilization/Intracytoplasmic Sperm Injection) protocols, switched the medicine, didn’t come back for follow-up, had more than 12 ARMS (Adherence to Refills and Medication) score etc. Inclusion criteria for study was: Patients (1) aged between 18-43 yrs, (2) diagnosed with PCOS as per the Rotterdam criteria (existence of any 2 of the following): (i) oligo-anovulation (a cycle length of >35 days or amenorrhea), (ii) clinical hyperandrogenism (hirsutism recorded as m-FG score of ≥ 6 with/without acne or androgenic alopecia) and (iii) Polycystic Ovaries (PCO) on ultrasound, (3) on metformin, pioglitazone with myoinositol and ethinylestradiol cyproterone treatment were included in the study. Patients with hypothyroidism, hyper-prolactinemia, renal (adrenal insufficiency) or history of laparoscopy and IVF/ICSI before starting study were excluded from the study. All participated patient gave an informed consent for their willingness to participate in the study. The questionnaires were filled via face-to-face interview with each participant at the time of their first visit and after 3 months of treatment. All participants completed all study questionnaires and demographic questions on age, highest education, occupation, marital status, disease duration, comorbidities, exercise behavior, family history and clinical features. Patients were assessed for BMI, PCOSspecific clinical features at each visit.
After diagnosis of PCOS, metformin was being administered 500 mg TID for three months, ethinylestradiol 0.035 mg OD for three months and pioglitazone 45 mg OD for 3 months.
Study measurements
BMI was calculated by using weight to height ratio and categorized as ≤ 25, >25 and >30 kg/m2 representing normal weight, overweight and obese patients, respectively. Permission for pre validated questioners was obtained from all respective researchers. Ferriman Gallway score was used to evaluate hirsutism [22]. PCOSQ is a disease specific questioner and it was used to assess quality of life of women with PCOS and improvement in quality of life. Chi-PCOSQ is Chinese version of PCOSQ with additional acne and hair loss domain. It consists of 30 questions with 7 no. scale i.e. 1 for maximum impairment and 7 for no impairment. It consists of 6 domains: emotions (7 items), hair growth (5 items), body weight (5 items), infertility (5 items), menstruation (4 items) and hair loss and acne (4 items) [23]. Its validity score was reliable and showed validity in Chinese women suffering from PCOS.
Patient’s adherence to medications assessed by using Adherence to Refills and Medication (ARMS) Scale. It was a 7 question scale with score of 1 for full adherence and 4 for non- adherence. We included patients with less than 12 ARMS scale score and all others were excluded from the study. This scale was validated in 435 chronic disease patients in which it shows Cronbach’s α=0.814 and when correlated with Mo risky scale it gives Spearman’s rho= -0.651, p <0.01, and it correlated more strongly with measures of adherence than the Mo risky scale [24].
Statistical analysis
Descriptive analysis was done to analyze the demographics data of the study sample and ANOVA and t-test were used to measure relation between different variables and stratified groups. Similarly, by using ANOVA we did comparison of frequency measures of different clinical features and QOL measures with drugs. Study patients were stratified by BMI (Body Mass Index: overweight vs. normal), infertility and family history of diabetes mellitus. Outcome of treatments after 3 months was assessed by chi-square and paired-t tests. The IBM SPSS 22.0 was utilized for all above mentioned analyses.
Results
Total 160 women meeting the inclusion criteria were included in the study. Out of these after 3 months 120 patients meet criteria for follow-up. Majority of patients were 25.4 ± 6.0 yrs old; 20.6% were obese, 61.2% infertile, and 46.2% patient’s presents with family diabetes history. Most of married patients were obese and overweight. Overweight patients BMI was significantly more than normal weight patients. Patients with family history of DM have significantly more BMI (28.2 ± 5.5) as compared to patients with no family DM history. Whereas overweight patients were married from longer duration than normal weight patients.
Majority of patients were not doing exercise. PCOS patients suffer from many co-morbidities while, in our data most of patients have history of hypotension (23.1%) and 14.4 % infertile patients had history of miscarriage. PCOS patients have many clinical features i.e. hirsutism 28.8%, oligomenorrhea (70.6%), menorrhagia (7.5%), acne (45.6%) and hair loss (58.8%). While in comparison of groups, more no. of overweight patients have hirsutism as compared to normal weight. The details of demographics and patient characteristics are given in Table 1 as demographics and baseline findings of PCOS patients.
| Subgroups | ||||||
|---|---|---|---|---|---|---|
| Characteristics | Normal Weight | Overweight | Obese | Infertility | Family history of Diabetes Mellitus | |
| BMI | All (n=160) | (BMI <25 kg/m2 | (BMI 25-30 kg/m2) | (BMI ≥ 30 kg/m2) | (n=98) | (n=74) |
| N Value | (n=61) | (n = 66) | (n = 33) | |||
| Mean | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Age | 25.4 ± 6.0 | 22.6 ± 4.6 | 26.3 ± 5.4 | 28.7 ± 7.14 | 27.3 ± 5.9 | 26.6 ± 6.5 |
| BMI | 26.9 ± 5.2 | 22.1 ± 2.1 | 27 ± 1.5** | 34.4±4.3 | 28.7 ± 5.1 | 28.2 ± 5.5* |
| mF-G score | 5.2 ± 3.35 | 6.0 ± 3.8) | 5.0 ± 2.8 | 4.1 ± 3.1 | 4.6 ± 2.9 | 5.3 ± 3.3 |
| Disease Duration(Year) | 4.9 ± 4.7 | 3.0 ± 2.6 | 4.8 ± 4.4* | 8.4 ± 6.3 | 6.4 ± 4.0 | 5.8 ± 5.25* |
| Marriage Duration(Year) | 6.7 ± 5.1 | 5.0 ± 4.0 | 6.0 ± 4.1* | 9.4 ± 6.4 | 6.5 ± 5.1 | 7.8 ± 5.2* |
| Mean | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD |
| Lab Findings | ||||||
| FSH (mIU/mL) | 5.3 ± 1.91 | 5.1 ± 2.1 | 5.3 ± 1.8 | 5.3 ± 1.5 | 5.3 ± 2.0 | 5.1 ± 1.7 |
| LH(mIU/mL) | 6.9 ± 3.84 | 7.3 ± 3.6 | 6.9 ± 4.1 | 6.2 ± 3.3 | 6.3 ± 3.9 | 6.4 ± 3.0 |
| Prolactin(ng/mL) | 17 ± 6.0 | 17.4 ± 5.4 | 16.2 ± 6.4 | 17.8 ± 6.0 | 17.1 ± 7.4 | 17.3 ± 5.8 |
| TSH ( μIU/mL) | 1.9 ± 1.04 | 1.7 ± 0.6* | 2.2 ± 1.4 | 2.0 ± 0.6 | 2.1 ± 1.2 | 2.1 ± 0.96 |
| n% | n% | n% | n% | n% | n% | |
| Exercise | ||||||
| No Exercise | 97 (60.6) | 36 (59.0) | 40 (66.6) | 21 (63.6) | 59 (62.2) | 48 (64.9) |
| 5-10 Minutes | 25 (15.6) | 7 (11.5) | 14 (21.2) | 4 (12.1) | 15 (15.3) | 11 (14.9) |
| 10-20 Minutes | 19 (11.9) | 9 (14.8) | 5 (7.6) | 5 (15.2) | 11 (11.2) | 8 (10.8) |
| 20 Or More | 19 (11.9) | 9 (14.8) | 7 (10.6) | 3 (9.1) | 13 (13.2) | 7 (9.5) |
| Comorbidities n% | ||||||
| Diabetes Mellitus | 2 (1.3) | 0 (0.0) | 2 (3.0) | 0 (0.0) | 1 (1.0) | 2 (2.7) 8 (10.8) 0 (0.0) |
| Hypertension | 12 (7.5) | 3 (4.9) | 4 (6.10) | 5 (15.2) | 10 (10.2) | 1 (1.4) |
| Dyslipidemia | 2 (1.3) | 0 (0.0) | 0 (0.0) | 2 (6.1) | 2 (2.0) | 9 (12.2) |
| Heart Disease | 2 (1.3) | 1 (1.6) | 0 (0.0) | 1 (3.0) | 2 (2.0) | 1 (1.4) |
| Emotional Depression | 16 (10) | 2 (3.3) | 9 (13.6) | 5 (15.2) | 12 (12.2) | 10 (13.5) 21 (28.4) |
| Endometriosis | 3 (1.9) | 0 (0.0) | 2 (3.0) | 1 (3.0) | 3 (3.1) | 1 (1.4) |
| Miscarriage History | 16 (14.4) | 3 (4.9) | 10 (15.2) | 3 (9.1) | 11 (11.2)* | 10 (13.5) |
| Hypotension | 37 (23.1) | 15 (24.6) | 10 (15.2) | 12 (36.4) | 20 (20.4) | 21 (28.4) |
| Clinical Features n% | ||||||
| Hirsutism | 46 (28.8) | 22 (36.1) | 20 (30.3)* | 4 (12.1) | 23 (23.5) | 23 (31.1) |
| Pelvic Pain | 73 (45.6) | 26 (42.6) | 30 (45.5) | 17 (51.5) | 45 (45.9) | 30 (40.5) |
| Oligo Menorrhea | 113 (70.6) | 46 (75.4) | 43 (65.2) | 24 (72.7) | 64 (65.3) | 52 (70.3) |
| Menorrhegea | 12 (7.5) | 7 (11.5) | 5 (7.6) | 0 (0.0) | 3 (3.1) | 2 (2.7) |
| Acne | 73 (45.6) | 31 (50.8) | 29 (43.9) | 13 (39.4) | 43 (43.9) | 28 (37.8)* |
| Hair Loss | 94 (58.8) | 22 (36.1) | 29 (43.9) | 15 (45.5) | 46 (46.9) | 28 (37.8) |
| Darkened Skin | 41 (25.6) | 15 (24.6) | 14 (21.2) | 12 (36.4) | 22 (22.4) | 16 (21.6) |
| Ultrasound Findings n% | ||||||
| Unilateral PCOS | 41 (25.6) | 22 (36.1) | 13 (19.7) | 6 (18.2) | 27 (27.6) | 18 (24.3) |
| Bilateral PCOS | 115 (71.9) | 39 (63.9) | 50 (75.8) | 26 (78.8) | 67 (68.4) | 539 (71.6) |
Abbreviations: BMI: Body Mass Index; BP: Blood Pressure; LH: Luteinizing Hormone; FSH: Follicle Stimulating Hormone; E2: Estradiol; PRL: Prolactin; TT: Total Testosterone; mF-G: Modified Ferriman-Gallwey; SD: Standard Deviation. *p < 0.05; **p < 0.001.
Table 1 Demographics and baseline findings of PCOS patients.
Overall chi-PCOSq score was 98.4 ± 24.2 with the lowest score in infertility domain i.e. 10.3 ± 7.9 in weight domain. Within BMI categories obese and overweight patients respectively had low score in weight, infertility and total score as compared to normal weight patients with p=0.000. Infertile patients were having low emotions, infertility and total chi-PCOSq score as compared to fertile group with p=0.03, p=0.00, p=0.004, respectively. In patients with family diabetes history within weight domain chi- PCOSq score was significantly different as compared to patients with no family diabetes history i.e. p=0.027. All other details of chi-PCOSq score of PCOS patients are given in Table 2 as Baseline HRQoL scores of PCOS patients.
| Characteristics | Groups | |||||
|---|---|---|---|---|---|---|
| Chi-PCOSq | All (N=160) | Normal Weight | Overweight | Obese | Infertility | Family history of Diabetes Mellitus |
| (BMI < 25 kg/m2 | (BMI 25-30 kg/m2) | (BMI ≥30 kg/m2) | __ | __ | ||
| (n=61) | (n = 66) | (n = 33) | (n=98) | (n=74) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Total score (30-210) | 98.4 ± 24.2 | 109.6 ± 22.7 | 92.2 ± 22.5** | 90 ± 22.4 | 90.4 ± 22.3* | 100.9 ± 24.0 |
| Weight domain (5-35) | 14.3 ± 10.5 | 21.5 ± 12.1 | 10.8 ± 6.2** | 7.9 ± 4.7* | 12.0 ± 8.2* | 12.3 ± 9.1* |
| Body hair domain (5-35) | 19.0 ± 8.5 | 19.6 ± 8.7 | 17.7 ± 7.8 | 20.4 ± 9.5 | 18.3 ± 8.4 | 18.8 ± 8.2 |
| Infertility domain (5-35) | 10.3 ± 7.9 | 14.4 ± 7.9 | 8.0 ± 6.7** | 7.4 ± 6.9 | 4.9 ± 3.4** | 9.6 ± 7.74 |
| Menstrual domain (4-28) | 15.7 ± 6.0 | 14.7 ± 5.4 | 16.2 ± 6.4 | 16.5 ± 6.2 | 16.8 ± 6.4 | 15.6 ± 6.4 |
| Emotions domain (7-49) | 22.4 ± 6.2 | 23.3 ± 5.4 | 22.6 ± 6.7 | 20.2 ± 6.1 | 21.3 ± 6.0 | 21.9 ± 6.6 |
| Acne & Hair loss domain (4-28) | 16.6 ± 6.7 | 16.0 ± 7.0 | 16.8 ± 6.9 | 17.4 ± 5.9 | 16.9 ± 6.9 | 17.1 ± 6.1 |
Table 2 Baseline HRQoL scores of PCOS patients.
Average ARMS score of patients included in follow-up study was 7.56 ± 1.14 and we included patients with more than 12 ARMS score in study. Metformin significantly reduced acne, oligomenorrhea, infertility and BMI with p=0.002, 0.001, 0.004 and 0.000 respectively. It improved weight, menstrual, acne and hair loss Chi-PCOSQ domain score with p=0.000 and emotions domain score with p=0.002.
While, in case of pioglitazone+myoinositol hair loss, acne, pelvic pain and oligomenorrhea symptoms were improved with p=0.001, 0.01, 0.007, 0.031 respectively. It improved weight, depression, menstrual, acne and hair loss, and total Chi-PCOSQ scores too. Ethinylestradiol cyproterone improved acne (p=0.006) and menorrhagia (p=0.010) symptoms. It also significantly improved emotions, menstrual, acne and hair loss domain scores with no effect on infertility and weight domains. Details of treatment versus clinical features and Chi-PCOSQ scores in visit 1 representing baseline data and visit 2 for followup data are represented in Table 3 and regression analysis for treatment outcome is given in Table 4.
Changes in clinical features and HRQOL of PCOS patients with different treatments (Table 3).
| Characteristics | Metformin | pioglitazone+myoinositol | Ethinylestradiol cyproterone |
|---|---|---|---|
| n=40 | n=38 | n=42 | |
| Hirsutism n% | |||
| (visit 1) | 17 (42.5) | 8 (21.0) | 18 (42.8) |
| (visit 2) | 17 (42.5) | 8 (21.0) | 18 (42.8) |
| Hair loss | |||
| (visit 1) | 34 (85.0) | 28 (73.6) | 39 (92.8) |
| (visit 2) | 29 (72.5) | 23 (60.5)** | 28 (66.6) |
| Acne | |||
| (visit 1) | 23 (54.7) | 8 (21.0) | 27 (64.3) |
| (visit 2) | 11 (27.5)** | 5 (13.1)** | 13 (35.1)* |
| Pelvic pain | |||
| (visit 1) | 12 (30.8) | 12 (31.5) | 22 (52.3) |
| (visit 2) | 12 (30.8) | 7 (18.4)** | 9 (21.4) |
| Oligomenorrhea | |||
| (visit 1) | 32 (80.0)** | 17 (43.5) | 29 (92.8) |
| (visit 2) | 23 (57.5) | 7 (18.4)* | 4 (9.5) |
| Menorrhegea | |||
| (visit 1) | 3 (7.5) | 1 (4.3) | 4 (11.9) |
| (visit 2) | 0 (0.0) | 1 (4.3) | 3 (7.1)* |
| Infertility | |||
| (visit 1) | 17 (42.3) | 38 (100.0) | 9 (75.0) |
| (visit 2) | 17 (42.3) | 31 (80.7) | 9 (75.0) |
| Darkened skin | |||
| (visit 1) | 13 (34.6) | 5 (13.0) | 9 (21.4) |
| (visit 2) | 4 (11.5) | 0 (0.0) | 9 (21.4) |
| Mean ± SD | Mean ± SD | Mean ± SD | |
| BMI | |||
| (visit 1) | 26.5 ± 3.8 | 29.8 ± 5.0 | 24.1 ± 3.9 |
| (visit 2) | 25.9± 3.9* | 28.6 ± 3.9 | 24.6 ± 4.8 |
| Mfg_score | |||
| (visit 1) | 5.4 ± 3.4 | 4.8 ± 2.3 | 6.1 ± 2.8 |
| (visit 2) | 5.6 ± 3.7 | 4.8 ± 2.9 | 6.5 ± 3.1 |
| Weight domain (5-35) | |||
| (visit 1) | 8.6 ± 4.8 | 10.0 ± 4.5 | 21.0 ± 12.1 |
| (visit 2) | 12.2 ± 5.9** | 13.7 ± 4.4* | 21.8 ± 11.9 |
| Body hair domain (5-35) | |||
| (visit 1) | 19.9 ± 8.1 | 19.6 ± 7.7 | 16.5 ± 8.0 |
| (visit 2) | 19.9 ± 8.1 | 20.5 ± 8.2 | 18.3 ± 8.0 |
| Infertility domain (5-35) | |||
| (visit 1) | 12.3 ± 8.1 | 3.8 ± 1.2 | 16.0 ± 7.2 |
| (visit 2) | 13.4 ± 8.4 | 4.9 ± 4.3 | 15.7 ± 7.6 |
| Menstrual domain (4-28) | |||
| (visit 1) | 14.3 ± 6.1 | 19.9 ± 5.3 | 13.0 ± 5.0 |
| (visit 2) | 17.8 ± 6.8** | 24.7 ± 4.0* | 22.1 (4.7)* |
| Emotions domain (7-49) | |||
| (visit 1) | 24.1 ± 8.1 | 21.6 ± 6.9 | 22.7 ± 4.4 |
| (visit 2) | 26.8 ± 8.3** | 25.0 ± 8.5* | 31.7 ± 7.1* |
| Acne & Hair loss domain (4-28) | |||
| (visit 1) | 15.5 ± 7.2 | 19.2 ± 6.2 | 14.8 ± 5.3 |
| (visit 2) | 19.8 ± 6.2** | 22.4 ± 5.0* | 21.1 ± 4.0* |
| Total chi_PCOSq score (30-210) | |||
| (visit 1) | 94.8 ± 28.4 | 94.1 ± 17.5 | 104.1 ± 26.6 |
| (visit 2) | 109.7 ± 27.9 | 111.3 ± 20.4* | 130.7 ± 30.9 |
Note Visit 1 is 1st hospital visit of PCOS diagnosed patients represented as baseline. Visit 2 represents the follow-up after taking 3 months treatment. The analysis was adjusted for the effect of medication adherence by using ARMS (Adherence to Refills and Medication Scale).
Abbreviation: HRQoL: Health Related Quality of life
Table 3 Changes in clinical features and HRQOL of PCOS patients with different treatments.
| Chi-PCOSq | Drugs | |||||
|---|---|---|---|---|---|---|
| Metformin | Pioglitazone+myoinositol | Ethinylestradiol cyproterone | ||||
| Coefficient (SE) | Coefficient (SE) | Coefficient (SE) | ||||
| Total score | ||||||
| Visit 2 (reference=visit 1) | 0.86 (0.09)* | 0.81 (0.18)** | 0.93 (0.09)** | |||
| Weight domain | ||||||
| Visit 2 (reference=visit 1) | 0.91 (0.15)** | 0.69 (0.09)** | 0.94 (0.05)** | |||
| Infertility domain | ||||||
| Visit 2 (reference=visit 1) | 0.84 (0.12)** | 1.73 (0.64)* | 1.01 (0.02)** | |||
| Menstrual domain | ||||||
| Visit 2 (reference=visit 1) | 0.93 (0.14) | 0.35 (0.13)* | 0.50 (0.16)** | |||
| Emotions domain | ||||||
| Visit 2 (reference=visit 1) | 0.70 (0.15)** | 0.83 (0.19)** | 0.25 (0.24) | |||
| Body hair domain | ||||||
| Visit 2 (reference=visit 1) | 0.92 (0.07)** | 0.99 (0.096) | 0.84 (0.09)** | |||
| Acne and hair loss domain | ||||||
| Visit 2 (reference=visit 1) | 0.76 (0.08)** | 0.67 (0.09)** | 0.35 (0.09)** | |||
*p<0.05; **p<0.005
Table 4 Regression analysis for different treatment effects on PCOS-specific quality of life by using Chi-PCOSQ.
Regression analysis for different treatment effects on PCOSspecific quality of life by using Chi-PCOSQ (Table 4).
Discussion
No research is available on quality of life and treatment outcomes in Pakistani women. In our study, we found that clinical features and depression due to PCOS led to reduction in QOL score. Proper treatment selection can lead to improvement in patient symptoms and HRQoL. HRQoL is nowadays measure of therapeutic outcomes. As, by our study metformin was more effective in reducing BMI and infertility. While, ethinylestradiol cyproterone was more effective in improving acne and menorrhagia symptoms significantly, with more serious menorrhagia side effects in some patients. Only, few studies [25- 29] are available on HRQoL improvement after treatment and no one in Pakistani women.
In 2006, a study conducted in Germany declared that metformin can improve patients, acne, BMI, menstrual disturbances and emotional distress which significantly correlates with our findings [26]. Other studies have also shown that metformin can improve clinical signs of acne in PCOS patients [30,31], which strongly correlates our findings where patients improved acne and HRQOL score too. A study reported that metformin increases ovulation rate leading to improvement in infertility rate. Studies conducted on metformin and troglitazone supports that these drugs can alleviate infertility issues of PCOS patients and improve pregnancy rate while in our study no significant values were observed [32,33]. Another study provides evidence that metformin only is not sufficient to counter PCOS symptoms so, it should be managed with lifestyle modifications [21]. Another study presents that 72.16% possess oligomenorrhea symptoms and most of infertile patients presents with this symptom which strongly correlates our findings according to which 65.3% patients with infertility have oligomenorrhea [10] and it was significantly improved by pioglitazone and ethinylestradiol cyproterone. Another study shows that 30% of women with PCOS will have normal menstruation [34]. Hirsutism presentation was reported in a study was about in 70% of women with PCOS [35]. However, in our study only 28.8% patients were presenting hirsutism clinical features. This can be due to PCOS clinical presentation differences among different ethnic groups. While, another study reported that 52% had clinical evidence of cutaneous hyperandrogenism and/or oligomenorrhea [36].
In a study about oral contraceptives impact on PCOS patient’s QoL in 2012, it was reported that oral contraceptives improved emotions and hirsutism domain score. While in our study, emotions domain score was improved significantly, with no effect on hirsutism [25].
As reported in many studies, our study also revealed that ultrasound is one of significant measure in diagnosis [37-45]. Gastrointestinal disturbances due to vitamin B12 malabsorption are one of most common adverse drug reaction of metformin [46-52]. While, in our study 11.5% patients on metformin show GIT related adverse reactions with other symptoms like hypoglycemia and increased urine frequency in few patients.
Importance of study findings to clinicians and patient care
High prevalence of PCOS and variation in prevalence of symptoms presentation in ethnic Pakistani women requires specific research in Pakistan. HRQOL being one of the latest health assessment tools in modern days would be the best measure to assess disease burden and treatment outcomes. As we included multiple drugs in our study. Hence, it can be a baseline study to give an idea to healthcare professionals to treat clinical signs specific treatment selection.
Limitations of study
Firstly, it was an observational study not a clinical trial. Secondly, HRQOL questioners were filled by patients so, self-reporting bias will definitely be there. Thirdly, in Pakistan treatment in all centers was prescribed for 3 months only so, we didn’t observe changes in hirsutism features of PCOS patients, as it requires a treatment of at least 6 months.
Conclusion
This is the first study in Pakistan to assess PCOS patients HRQOL and treatment outcomes. Our, result can provide an important baseline data to further clinical associations and can pave a way to decisive guidelines for Pakistani PCOS patients. Further, studies on large scale are required to assess treatment outcomes and changes in HRQOL of PCOS patients.
References
- World Health Organisation (2014) WHO | WHOQOL: Measuring Quality of Life.
- Cella D, Wagner L, Cashy J, Hensing TA, Yount S (2007) Should health-related quality of life be measured in cancer symptom management clinical trials? Lessons learned using the functional assessment of cancer therapy. J Natl Cancer Inst Monogr 37: 53-60.
- Osoba D (1999) What has been learned from measuring health-related quality of life in clinical oncology. Eur J Cancer 35: 1565-1570.
- US (2009) Department of Health and Human Services F and DA for DE and R. Guidance for Industry Use in Medical Product Development to Support Labeling Claims Guidance for Industry.
- Mohamed-Hussein ZA, Harun S (2009) Construction of a polycystic ovarian syndrome (PCOS) pathway based on the interactions of PCOS-related proteins retrieved from bibliomic data. Theor Biol Med Model 6: 1-7.
- Teede H, Deeks A, Moran L (2010) Polycystic ovary syndrome: A complex condition with psychological, reproductive and metabolic manifestations that impacts on health across the lifespan. BMC Med 1: 8.
- Ramezani Tehrani F, Simbar M, Tohidi M, Hosseinpanah F (2011) The prevalence of polycystic ovary syndrome in a community sample of Iranian population: Iranian PCOS prevalence study. Reprod Biol Endocrinol 9:39.
- Fauser BCJM (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81: 19-25.
- March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, et al. (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25: 544-551.
- Sakthi V, Tucker S, Autonomous C (2017) Polycystic ovaries syndrome (PCOS) among infertile female in Ramanathapuram, Tamil Nadu. 2: 262-264.
- Azziz R, Adashi EY (2016) Stein and Leventhal: 80 years on. Am J Obstet Gynecol 214: 247-247e1.
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, et al. (2016) The management of anovulatory infertility in women with polycystic ovary syndrome: An analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 22: 687-708.
- Sirmans SM, Pate KA (2013) Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin Epidemiol 6: 1-13.
- National Institutes Of Health (2012) Evidence-based Methodology Workshop on Polycystic Ovary Syndrome.
- Nestler JE (2008) Metformin for the treatment of the polycystic ovary syndrome. N Engl J Med 358: 47-54.
- Melo A, Ferriani R, Navarro P (2015) Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics 70: 765-769.
- Thessaloniki T, Pcos EA, Workshop C, March G (2008) Consensus on infertility treatment related to polycystic ovary syndrome. Fertil Steril 89: 505-522.
- Sun X, Xu J, Hao C, Ren C, Zhang Y, Chen S, et al. (2010) Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome. Nat Publ Gr 43: 55-59.
- Escobar-Morreale HF, Luque-Ramírez M, San Millán JL (2005) The molecular-genetic basis of functional hyperandrogenism and the polycystic ovary syndrome. Endocr Rev 26: 251-282.
- Kalro B, Loucks (2001) Neuromodulation in polycystic ovary syndrome. Obstet Gynecol
- Zhao Y, Qiao J (2013) Ethnic differences in the phenotypic expression of polycystic ovary syndrome. Steroids 78: 755-760.
- Ferriman D, Gallwey JD (1961) Clinical Assessment of Body Hair Growth in Women. J Clin Endocrinol Metab: 1440-1447.
- Ou H, Wu MH, Lin CY, Chen PC (2015) Development of Chinese Version of Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (Chi-PCOSQ). PLoS One 10: e0137772.
- Kripalani S, Risser J, Gatti ME, Jacobson TA (2009) Development and evaluation of the Adherence to Refills and Medications scale (ARMS) among low-literacy patients with chronic disease. Value Heal 12: 118–123.
- Cinar N, Harmanci A, Demir B, Yildiz BO (2012) Effect of an oral contraceptive on emotional distress, anxiety and depression of women with polycystic ovary syndrome: A prospective study. Hum Reprod 27: 1840-1845.
- Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, et al. (2006) Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality. Hum Reprod 21: 1925-1934.
- Harris-Glocker M, Davidson K, Kochman L, Guzick D, Hoeger K (2010) Improvement in quality-of-life questionnaire measures in obese adolescent females with polycystic ovary syndrome treated with lifestyle changes and oral contraceptives, with or without metformin. Fertil Steril 93: 1016-1019.
- Moll E, Van Wely M, Lambalk CB, Bossuyt PMM, Van Der Veen F (2012) Health-related quality of life in women with newly diagnosed polycystic ovary syndrome randomized between clomifene citrate plus metformin or clomifene citrate plus placebo. Hum Reprod 27: 3273-3278.
- Ou HT, Chen PC, Wu MH, Lin CY (2016) Metformin improved health-related quality of life in ethnic Chinese women with polycystic ovary syndrome. Health Qual Life Outcomes 14: 119.
- Genazzani AD, Lanzoni C, Ricchieri F, Baraldi E, Casarosa E, et al. (2007) Metformin administration is more effective when non-obese patients with polycystic ovary syndrome show both hyperandrogenism and hyperinsulinemia. Gynecol Endocrinol 23: 146–52.
- Palomba S, Falbo A, Zullo F, Orio F (2009) Evidence-based and potential benefits of metformin in the polycystic ovary syndrome: A comprehensive review. Endocr Rev 30: 1-50.
- Sivalingam VN, Myers J, Nicholas S, Balen AH, Crosbie EJ (2014) Metformin in reproductive health, pregnancy and gynaecological cancer: Established and emerging indications. Hum Reprod Update 20: 853–68.
- Guyatt G, Weaver B, Cronin L, Dooley JA, Azziz R (2004) Health-related quality of life in women with polycystic ovary syndrome, a self-administered questionnaire, was validated. J Clin Epidemiol 57: 1279-1287.
- Balen AH, Conway GS, Kaltsas G, Techatraisak K, Manning PJ, et al. (1995) Poly cystic ovary syndrome: The spectrum of the disorder in 1741 patients. Hum Reprod 10: 21-27.
- Fauser BCJM, Tarlatzis BC, Rebar RW, Legro RS, Balen AH, et al. (2012) Consensus on women’s health aspects of polycystic ovary syndrome (PCOS). Hum Reprod 27: 14-24.
- Conn JJ, Jacobs HS, Conway GS (2000) The prevalence of polycystic ovaries in women with type 2 diabetes mellitus. Clin Endocrinol (Oxf) 52: 81-86.
- Yao G, Chung CW, Yu CF, Taiwan W-B, Wang J-D, et al. (2002) Development and verification of validity and reliability of the WHOQOL-BREF Taiwan Version. J Formos Med Assoc 101: 342-351.
- Abdel Razek AAK, El-Basyouni SR (2016) Ultrasound of knee osteoarthritis: Interobserver agreement and correlation with Western Ontario and McMaster Universities Osteoarthritis. Clin Rheumatol 35: 997-1001.
- Razek A, Fouda NS, Elmetwaley EE (2009) Sonography of the knee joint. J Ultrasound 12: 53-60.
- Razek AAKA, Al Mahdy Al Belasy F, Ahmed WMS, Haggag MA (2015) Assessment of articular disc displacement of temporomandibular joint with ultrasound. J Ultrasound 18: 159-163.
- Ting RZ-W (2006) Risk Factors of Vitamin B12 Deficiency in Patients Receiving Metformin. Arch Intern Med 166.
- National Institutes Of Health Evidence-based Methodology Workshop on Polycystic Ovary Syndrome (2012).
- Abdel Razek AAK, El-Basyouni SR (2016) Ultrasound of knee osteoarthritis: Interobserver agreement and correlation with Western Ontario and McMaster Universities Osteoarthritis. Clin Rheumatol 35: 997–1001.
- Abdel Razek EE, Fouda NS, Elmetwaley N (2009) Sonography of the knee joint. J Ultrasound 12: 53–60.
- Razek AAKA, Al Mahdy Al Belasy F, Ahmed WMS, Haggag MA (2015) Assessment of articular disc displacement of temporomandibular joint with ultrasound. J Ultrasound18: 159–163.
- Borghi L, Leone D, Vegni E, Galiano V, Lepadatu C, et al. (2018) Psychological distress anger and quality of life in polycystic ovary syndrome : Associations with biochemical, phenotypical and socio-demographic factors. J Psychosom Obstet Gynecol 39: 128-137.
- Hahn S, Benson S, Elsenbruch S, Pleger K, Tan S, et al. (2006) Metformin treatment of polycystic ovary syndrome improves health-related quality-of-life, emotional distress and sexuality. Hum Reprod. 21: 1925–1934
- Ou H, Wu MH, Lin CY, Chen PC (2015) Development of Chinese Version of Polycystic Ovary Syndrome Health-Related Quality of Life Questionnaire (Chi-PCOSQ) 10: e0137772.
- Fallat ME, Siow Y, Marra M, Cook C, Carrillo A (1997) Mullerian-inhibiting substance in follicular fluid and serum: A comparison of patients with tubal factor infertility, polycystic ovary syndrome, and endometriosis. Fertil Steril 67: 962–965.
- Ferriman D, Gallwey JD (1961) Clinical Assessment of Body Hair Growth in Women. J Clin Endocrinol Metab 21: 1440-1447.
- Chen PC, Wu MH, Lin CY (2016) Metformin improved health-related quality of life in ethnic Chinese women with polycystic ovary syndrome. Health Qual Life Outcomes 14: 1-10.
- Kendra C (2014) What Is Longitudinal Research? The Pros and Cons of Longitudinal Research.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences