The Management of Sub-threshold Serum LH Post GnRH Agonist Trigger
Jigal Haas, Yaakov Bentov
1Toronto Centre for Advanced Reproductive Technology, Division of Reproductive Sciences, Toronto, Canada
2Department of Obstetrics and Gynecology, University of Toronto, Canada
3Samuel Lunenfeld Research Institute, Mount Sinai Hospital, Toronto, Canada
- *Corresponding Author:
- Yaakov Bentov
Senior REI Physician Tcart Fertility Partners
150 Bloor Street West, Suite 210
Toronto, ON M5S 2X9
Tel: 416.972.0110 (ext. 223)
Fax: 416.972.0036
E-mail: ybentov@tcart.ca
Received date: October 09, 2015; Accepted date: October 13, 2015; Published date: October 20, 2015
Citation: Bentov Y. The Management of Sub-Threshold Serum LH Post GnRH Agonist Trigger. J Reproductive Endocrinol & Infert. 2015, 1:1. doi: 10.4172/JREI.100001
Copyright: © 2015 Bentov Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Editorial
Ovarian hyper stimulation syndrome (OHSS) is a life-threatening condition characterized by haemodynamic instability, haemoconcentration and hypoxia. The incidence OHSS has increased dramatically over the past three decades mainly because of the widespread use of ovulation inductions agents and assisted reproduction techniques.
Critical cases of OHSS may result in severe morbidity such as; thromboembolism, renal failure, hypoxemia and electrolyte imbalance, and mortality.
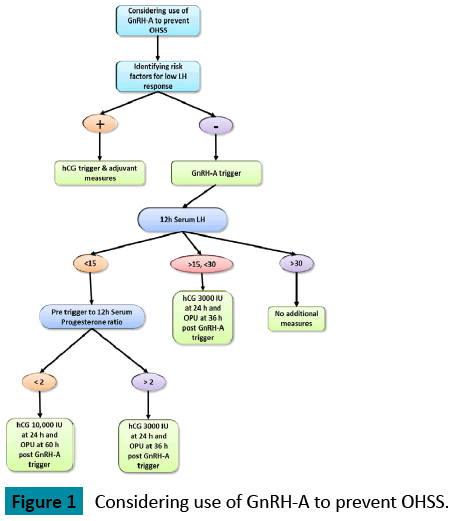
Several studies have shown that the use of GnRH agonist for triggering of final oocyte maturation reduces the risk for the development of OHSS even in patients at high risk of developing OHSS [1-4]. In contrast to HCG, the GnRH agonist-induced surge resembles the natural mid cycle surge of gonadotropins and exposes follicles to both LH and FSH. However, the mid cycle surge of gonadotropins after GnRH agonist triggering is shorter in duration and amplitude in comparison with a natural cycle [5,6]. It is still not fully understood how the use of GnRH agonist trigger reduces the risk for OHSS; One of the hypotheses is that the shorter half-life of the endogenous LH surge induced by GnRH agonist, compared with the continuous high levels of the longer acting HCG, both of which act by binding to the LH receptor, induces a shorter and milder secretion of vasoactive substances such as vascular endothelial growth factor (VEGF) known together with other pro inflammatory cytokines to play a fundamental role in the pathophysiology of OHSS [7,8]. The lack of a widespread acceptance of GnRH agonist triggering is at least in part due to a small subset of patients who do not respond to the GnRH agonist injection with an adequate LH surge and result in a poor or no oocyte yield. As shown in the Figure 1.
A rise in serum LH and progesterone after a GnRH trigger indicates that an endogenous flare has occurred and oocyte maturation has been initiated. Therefore the measurement of serum LH concentration twelve hours post GnRH agonist trigger became a common practice [9-11]. However, a threshold of post GnRH agonist trigger serum LH concentration is yet to be established. Several studies examined the relationship between the magnitude of the LH surge 12 hours after GnRH-a trigger and the yield of mature oocytes.
These studies reported on a range of serum LH threshold associated with a poor oocyte yield starting at serum LH level <15 IU/L and up to >52 IU/L. Serum LH level <15 IU/L were associated with a decreased number of mature oocytes and increased risk of empty follicle syndrome compared with patients with serum LH levels >15 and 12-hours post trigger [9,11,12]. Shapiro et al. [12] demonstrated a modest reduction in oocyte yield and maturity when the serum level of LH 12 hours after GnRH agonist trigger was less than 52 IU/L, and a dramatic reduction in yield and maturity was observed when that level was less than 12 IU/L.
A study by Chen et al. showed no improvement in oocyte yield when the serum level of LH >30 IU/L [10]. Moreover, there are no studies that address the question of how to manage patients that had a below threshold post GnRH agonist trigger serum LH. The dilemma that the managing physician is faced with is the following; if the sub-threshold LH represents an improper pituitary response, continuing with oocyte retrieval may result in no oocytes retrieved and the conduction of an unnecessary medical procedure. However, if the low serum LH was however sufficient to induce oocyte maturation and ovulation, deciding to administer a rescue HCG trigger and delaying oocyte retrieval may result in premature ovulation, no oocytes retrieved and development of OHSS. To make the dilemma even more complicated there is a third option; the rise in LH is adequate to induce oocyte maturation and ovulation of some of the follicles but not in others. We had recently reported on a patient that had an escape of GnRH antagonist suppression of ovulation that resulted in ovulation of a single follicle but yet allowed for a retrieval of 18 oocytes shortly after [14]. This report as well as others suggests that follicles differ in their sensitivity to the amplitude of the LH surge.
The following are strategies that are practiced in order to reduce the risk of poor oocyte yield post GnRH agonist trigger:
Identifying patients with a propensity for poor LH response:
Meyer et al. [11] aimed to identify risk factors for a suboptimal response to GnRH agonist trigger in IVF cycles. The authors identified prolonged use of contraception and very low endogenous serum LH concentration on the day of trigger as independent risk factors for a suboptimal response to GnRHagonist trigger. The authors suggested to avoid using GnRH agonist trigger in patients with preexisting hypothalamic-pituitary axis dysfunction and those with a pre-trigger LH<0.5 IU/L.
Using a dual trigger (GnRH agonist with HCG)
Shapiro et al. [13] retrospectively evaluated administration of low dose HCG (1000-2500 IU) in combination with GnRH agonist to induce final oocyte maturation in patients who had significant risk factors for OHSS. He named this combination the "dual trigger". The goals of using a dual trigger are to aid in oocyte maturation, while providing a more sustained support for the corpus luteum. He demonstrated high clinical pregnancy rate along with the absence of OHSS and concluded that the dual trigger is safe and effective for oocyte maturation in patients with significant risk factors for ovarian hyperstimulation syndrome.
Using serum progesterone as a surrogate marker to predict oocyte yield
A rise in serum LH and progesterone indicates that an endogenous flare has occurred and oocyte maturation has begun. Kummer et al. [9]. demonstrated that there is no post-trigger progesterone cut-off that can be used to reliably predict the number of oocytes retrieved, but all patients with empty follicles syndrome (EFS) had low serum LH and progesterone levels (<3.5 ng/ml). The measurement of post GnRHa trigger progesterone is therefore useful in counseling patients prior to oocyte retrieval regarding the likelihood of EFS and also in deciding appropriate timing of rescue hCG. In our clinic we measure the LH and progesterone levels at the morning of the trigger and 12 post trigger. if the ratio of progesterone level post trigger/ progesterone level before trigger is bellow 2 we add full dose hCG ( Pregnyl 10,000 IU) 24 hours post trigger and perform the retrieval 60 hours post the GnRH agonist trigger.
If the LH level is below 15 IU/L and the ratio of progesterone level before trigger/ progesterone levels post trigger is above 2, the oocyte maturation has been initiated and therefore we supplement low dose HCG (3000 IU pregnyl) to assist the oocyte maturation.
Adding an HCG injection the day before the OPU
If LH level 12 hours post trigger is >15 IU/L but <30 IU/L, the risk of EFS is extremely low but the risk of low oocyte yield is increased and therefore we recommend to supplement HCG (3000 IU pregnyl) in order to assist the oocyte maturation.
Conclusion
The use of GnRH agonist trigger significantly reduces the risk of OHSS. In most oocyte retrievals triggered with GnRH agonist the yield is comparable to HCG trigger. However, the small percentage of cases with a non-optimal oocyte yield could be managed by a better patient selection and by the use of thresholds of 12 hour post trigger LH and progesterone. These hormone concentrations can be used to guide the decision on whether to proceed with retrieval, switch to HCG trigger and postpone the retrieval or augment with a low dose HCG while keeping the original date of the retrieval.
References
- Humaidan PS, Kol S, Papanikolaou EG(2011)Gnrh agonist for triggering of final oocyte maturation: time for a change of practice? Hum Reprod Update17: 510-524.
- Engmann L, Siano L, Schmidt D, Nulsen J, Maier D, et al. (2006) GnRH agonist to induce oocyte maturation during IVF in patients at high risk of OHSS. Reprod Biomed Online 13: 639-644.
- Humaidan P Luteal (2009) Phase rescue in high-risk OHSS patients by GnRHa triggering in combination with low-dose HCG: a pilot study. Reprod Biomed Online 18: 630-634.
- Humaidan P, PapanikolaouEG,TarlatzisBC (2009) GnRH to trigger final oocyte maturation: a time to reconsider. Hum Reprod24: 2389-2394.
- Gonen Y, Balakier H, Powell W, Casper RF, et al. (1990) Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab 71: 918-922.
- Itskovitz J, Boldes R, Levron J, Erlik Y, Kahana L, et al. (1991)Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil Steril56: 213-220.
- Pellicer A, Albert C, Mercader A, Bonilla-Musoles F, Remohí J, et al. (1999) The pathogenesis of ovarian hyperstimulation syndrome: in vivo studies investigating the role of interleukin-1beta, interleukin-6, and vascular endothelial growth factor. Fertil Steril 71: 482-489.
- McClure N, Healy DL, Rogers PA, Sullivan J, Beaton L,et al. (1994) Vascular endothelial growth factor as capillary permeability agent in ovarian hyperstimulation syndrome. Lancet 344: 235-236.
- Kummer NE, Feinn RS, Griffin DW, Nulsen JC, Benadiva CA, et al.(2013) Predicting successful induction of oocyte maturation after gonadotropin-releasing hormone agonist(GnRHa)trigger. Hum Reprod 28: 152-159.
- Chen SL, Ye DS, Chen X, Yang XH, Zheng HY, et al. (2012) Circulating luteinizing hormone level after triggering oocyte maturation with GnRH agonist may predict oocyte yield in flexible GnRH antagonist protocol. Hum Reprod 27: 1351-1356.
- MeyerL, Murphy LA, Gumer A, Reichman DE, Rosenwaks Z,et al.( 2015) Risk factors for a suboptimal response to gonadotropin-releasing hormone agonist trigger during in vitro fertilization cycles. Fertil Steril 104: 637-642.
- Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, et al. (2011) Efficacy of induced luteinizing hormone surge after "trigger" with gonadotropin-releasing hormone agonist. Fertil Steril 95: 826-828.
- Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Thomas S, et al.(2008) Gonadotropin-releasing hormone agonist combined with a reduced dose of human chorionic gonadotropin for final oocyte maturation in fresh autologous cycles of in vitro fertilization. Fertil Steril 90: 231-233.
- Klement-Hersku and Bentov Y(2013). Ovulation escape in a GnRh antagonist IVF cycle is notan all or none phenomenon: a case report. Clin Med Insights Reprod Health. In press.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences