The Role of Microorganisms in Anabolic Steroids Transformation
Mohammad Yasin Mohammad
Faculty of Pharmacy, Middle East University, Amman-11831, Jordan and H.E.J. Research Institute of Chemistry, International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan
- *Corresponding Author:
- Mohammad Yasin Mohammad
Faculty of Pharmacy, Middle East University, Amman-11831, Jordan and H.E.J. Research Institute of Chemistry
International Center for Chemical and Biological Sciences, University of Karachi, Karachi-75270, Pakistan
E-mail: mhm17feb@hotmail.com
Received date: January 21, 2017; Accepted date: February 24, 2017; Published date: February 28, 2017
Citation: Mohammad MY, An Appraisal of the Assisted Reproductive Technologies that Contributed to Current Infertility Treatment. J Reproductive Endocrinol & Infert, 2017; 2:18. DOI: 10.4172/2476-2008.100018
Copyright: © 2017 Mohammad MY. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Short Communication
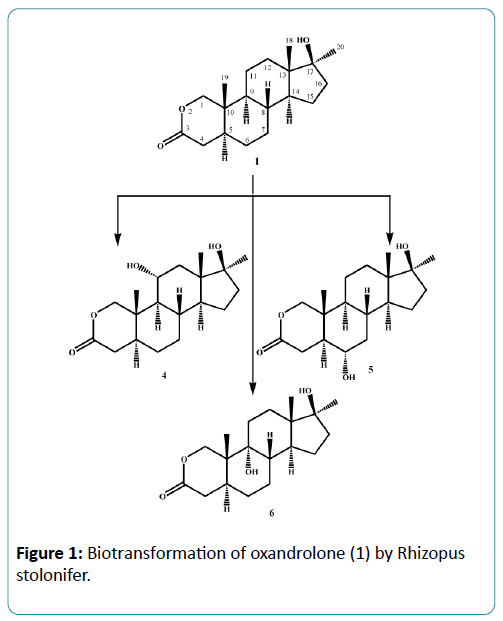
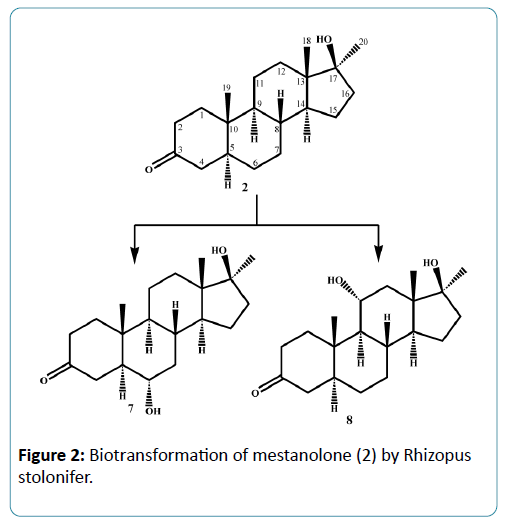
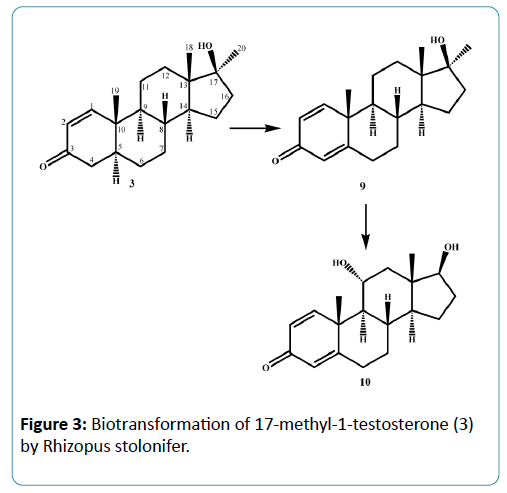
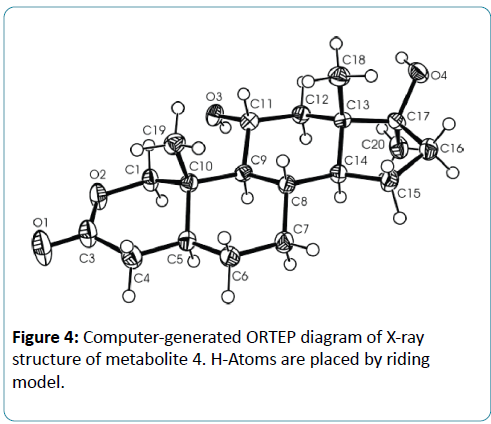
Microorganisms have been used extensively for hydroxylation of anabolic steroids since their enzymes can catalyze reactions with high regio-and stereospecifity. Their ability to oxidize steroidal compounds has immense synthetic and commercial importance. Selected anabolic steroids (oxandrolone (1), mestanolone (2) and 17-methyl-1-testosterone (3)) were subjected to biotransformation using the plant pathogen fungus Rhizopus stolonifer (NRRL 1392). Oxandrolone (1) and mestanolone (2) are anabolic synthetic derivatives of testosterone that act on androgen receptors [1]. Oxandrolone (1) has two main advantages, first it does not aromatize to oestrogen and second it does not significantly affect the production of testosterone in the body at a 10 mg dose. For this reason, it is considered to be the safest of all steroids. 17- Methyl-1-testosterone (3) is an anabolic synthetic derivative of mestanolone (2). Mestanolone (2) and 17-methyl-1-testosterone (3) are the main intermediates in the synthesis of oxandrolone (1). Incubation of oxandrolone (1) with R. stolonifer yielded metabolites 4-6 (Figure 1), [2]. While incubation of mestanolone (2) and 17-methyl-1-testosterone (3) with R. stolonifer produced metabolites 7, 8 (Figure 2) and 9, 10 (Figure 3) [3], respectively. Metabolites 4-10 were isolated and purified by the chromatographic techniques (TLC and HPLC), and their structures were deduced through comparative spectroscopic studies (1H-NMR, 13C-NMR, DEPT-135°, DEPT-90°, COSY 45°, HSQC, and HMBC) with substrates 1-3. The stereochemistry in compounds 4-8 and 10 were determined by NOESY spectrum and single-crystal X-ray diffraction studies for metabolite 4 (Figure 4). Compounds 4, 8 and 10 were obtained as major products with 25.0%, 11.4% and 18.0% yields, respectively. The percentage yields of metabolites 4-10 are summarized in Table 1.
| Metabolite | % Yield |
|---|---|
| 4 | 25.00% |
| 5 | 5.00% |
| 6 | 8.00% |
| 7 | 6.00% |
| 8 | 11.40% |
| 9 | 9.00% |
| 10 | 18.00% |
Table 1: Percentage yields of resulted metabolites from oxandrolone (1), mestanolone (2) and 17-methyl-1-testosterone (3).
In conclusion, microbial transformation by Rhizopus stolonifer proved to be effective for dehydrogenation, demethylation, and α-hydroxylation of C-6, C-9 and C-11. Metabolites 4, 8 and 10 were produced in good yields, which may be used to synthesize new compounds with interesting biological activities. The metabolites of oxandrolone (1) might be prescribed to promote muscle re-growth in patients with AIDS for the treatment of severe burn injury, trauma, chronic infections, neuromuscular disorders and alcoholic hepatitis.
References
- Pappo R, Jung JC (1962) 2-Oxasteroids: a new class of biologically active compounds. Tetrahedron Lett 3: 365-371.
- Choudhary MI, Mohammad MY, Musharraf SG, Parvez M, Al-Aboudi A, et al. (2009) New oxandrolone derivatives by biotransformation using Rhizopus stolonifer. Steroids 74: 1040-1044.
- Mohammad MY, Musharraf SG, Al-Majid AM, Atta-ur-rahman, Choudhary MI (2013) Biotransformation of mestanolone and 17-methyl-1-testosterone by Rhizopus stolonifer. Biocatalysis and Biotransformation 31: 153-159.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences