Analysis of the methylation rate of the androgen receptor promoter region in granulosa cells in moderate to severe endometriosis patients undergoing controlled ovarian hyperstimulation
Yoko Nakamura, Yoshiki Yamashita, Natsuho Saito, Hikaru Yamamoto, Masami Hayashi, Yoshito Terai, Masahide Ohmichi
1Department of Obstetrics and Gynecology, Osaka Medical College, Takatsuki, Osaka, Japan
2Mizayaki Ladies Clinic, Kita-ku, Osaka city, Osaka, Japan
- *Corresponding Author:
- Yoshiki Yamashita
M.D.,Department of Obstetrics and Gynecology
Osaka Medical College, 2-7Daigakumachi, Takatsuki
Osaka569-8686, Japan
Fax: +81-726-81-3723
E-mail: yoppy1205@gmail.com
Received date: November 07, 2015; Accepted date: December 03, 2015; Published date: December 28, 2015
Citation: Yamashita Y, Nakamura Y, SaitoN, et al. Analysis of the methylation rate of the androgen receptor promoter region in granulosa cells in moderate to severe endometriosis patients undergoing controlled ovarian hyperstimulation J Reproductive Endocrinol & Infert. 2015, 1:5. doi: 10.4172/JREI.100005
Copyright: © 2015 SaitoN, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: To investigate the effect of the methylation rate of the androgen receptor (AR) promoter region in granulosa cells in patients with endometriosisinduced infertility. Method: Eighteen patients with moderate to severe endometriosis and a control group of 24 patients without endometriosis who underwent assisted reproductive technologies (ART) were investigated. Genomic DNA was extracted from granulosa cells and subjected to bisulfite treatment. The methylation rate of the AR promoter region was compared between the endometriosis group and the control group, and the relationship between clinical outcome and the methylation rate of the AR promoter region was investigated using real-time methylation-specific polymerase chain reaction (MSP). The MSP data was confirmed using bisulfite sequencing. Results: Antral follicle count (AFC) and number of retrieved oocytes was lower in the endometriosis group than in the control group (p < 0.05). The methylation rate of the AR promoter region was statistically higher in the endometriosis group than in the control group; moreover, the methylation rate was negatively correlated with AFC and the number of retrieved oocytes. Conclusion: A higher methylation rate of the AR promoter region in granulosa cells could contribute to poor follicular development in endometriosis.
Keywords
Endometriosis, Granulosa cell, AR, Methylation, MSP
Introduction
The resulting poor outcome of in vitro fertilization-embryo transfer (IVF-ET) in patients with endometriosis is widely considered to be due to altered folliculogenesis [1], embryo toxicity against early embryonic development [2], and impaired fertilization [3]. Although endometriosis is still one of the most enigmatic of all gynecological diseases, recent epigenetic changes in the disease have gradually come under closer investigation [4,5]. Bulun speculated that genetic predisposition or the exposure to environmental toxins of fetal progenitor cells destined to form adult female pelvic organs may result in epigenetic events, including promoter hypomethylation and the overexpression of steroidogenic factor-1 (SF-1) and estrogen receptor-β (ER-β), both of which play critical roles in the pathogenesis of endometriosis [4]. In patients with endometriosis, non-methylation of the promoter region of ER-β results in the overexpression of ER-β and, consequently, the expression of ER-α and progesterone receptors (PRs) is suppressed [5].
The most notable stimulatory action of androgen is its ability to induce follicular differentiation by follicle-stimulating hormone (FSH) [6-8].
In contrast, however, androgen has also been reported to inhibit follicular differentiation and follicular atresia [9,10]. These opposing androgenic actions are mediated by a specific nuclear receptor, or androgen recepto. Granulosa cells in dominant follicles show stronger nuclear staining for androgen receptors than that found in thecal cells. Moreover, the staining intensity of AR is strongest in the early luteal phase just after ovulation and then gradually declines [11]. We previously reported that the expression of AR mRNA is positively correlated with FSHR mRNA in non-endometriosis patients; however, such correlation was not confirmed in patients with endometriosis. These results may imply that endometriosis has a negative effect on the interaction between AR and FSHR in granulosa cells and, therefore, we hypothesized that the promoter region of AR might be methylated in the presence of the disease [12]. However, there have been no reports so far quantitating the methylation rate of the AR promoter region in the granulosa cells of patients with endometriosis. Therefore, in this study, we performed realtime methylation-specific polymerase chain reaction (MSP) in order to investigate the effect of the methylation rate of the AR promoter region on IVF-ET outcome in patients with moderate to severe endometriosis.
Material and Methods
Patient population
Eighteen patients with moderate to severe endometriosis and a control group of 24 patients without endometriosis were recruited. All 42 patients in the study received ART between October 2010 and December 2013 at Osaka Medical College. When patients with endometriosis had undergone a laparoscopic bilateral endometriotic cystectomy, the diagnosis of moderate or severe endometriosis was confirmed based on the revised American Society of Fertility & Sterility (rAFS) score. Among those 18 patients with endometriosis, five cases were complicated due to deep infiltrating lesions. In all 24 patients in the control group, the absence of endometriosis was confirmed during laparoscopic surgery for an ectopic pregnancy within 1 year of receiving ART. All of the endometriosis patients had undertaken a laparoscopic bilateral endometriotic cystectomy and were diagnosed as having moderate to severe endometriosis using the revised American Society for Reproductive Medicine (rASRM) score. In all patients in the control group, the absence of endometriosis was confirmed when laparoscopic surgery for an ectopic pregnancy was carried out. The inclusion criteria were as follows: (i) 30-42 years of age with regular menstrual cycles and (ii) the absence of any endocrine disease. The excluding criteria were as follows: (i) evidence of postmenopausal FSH and estradiol levels and (ii) any suspicion of malignant ovarian disease.
The institutional review board approved this protocol (2012-135) and its consent form, and informed consent for this study was obtained from all participants.
Protocol for controlled ovarian hyperstimulation
Controlled ovarian hyperstimulation (COH), hormone assays, follicle monitoring, retrieval of oocytes, insemination, embryo culture, embryo transfers, and confirmation of embryo quality were performed as previously described [13]. Hormone assaying was carried out at the time of oocyte retrieval, and basic values for LH and FSH were assayed at the time of the antral follicle count, :"(AFC)" The number of antral follicles was counted at day 2 before starting hMG/FSH administration. Pregnancy was confirmed by the identification of an intrauterine gestational sac during ultrasound examination.
Genomic DNA preparation from granulosa cells
After oocyte retrieval, granulosa cells 18mm or larger in the aspirated follicular fluid from each patient were isolated and suspended in a tube according to the methods reported by Toya et al [14].
Genomic DNA was extracted from the granulosa cells shortly after retrieval of the oocyte using an Easy-DNA Kit™ (Life technologies, California, US) in accordance with the manual.
Real-time MSP and bisulfite genomic sequences
Bisulfite conversion of genomic DNA extracted from the granulosa cells was performed with the EZ DNA Methylation-Gold Kit (Zymo Research, California, US). Converted DNA was quantified prior to downstream PCR application, and the amount of DNA was determined by spectrophotometric measurement using the NanoDrop 2000 spectrophotometer (Thermo Scientific, Waltham, MA) [15]. Real-time MSP using SYBR Green was performed on the DNA from the granulosa cells in accordance with the manual. Real-time MSP reactions were performed on 20μl of each patient sample by OneStep realtime PCR (Perkin-Elmer Applied Biosystems, Tokyo, Japan). The mixture contained 500nM of each forward primer and reverse primer, 1xPOWER SYBR® Green PCR Master Mix (Life technologies, California, US), and 100 ng of DNA. To create standard curves of AR methylation and non-methylation, methyl and non-methyl DNA were synthesized (Life technologies, California, US). The primers for real-time MSP were designed and synthesized (Life technologies, California, US) as shown in Table 1. These primers were designed using Methylation Primer Express (Life technologies, California, US) to detect any methylated CpG islands of the AR promoter region. PCR amplicon was104bp in size and corresponded to GenBank accession number X78592 and nucleotides 5,860 to 5,963 (UCSC Genome Browser: chrX: 66,763,945 to 66,764,048; GRCh37/hg19). PCR Conditions were 95°C for 2 minutes, followed by 35 cycles of 95°C for 30s, 56°C for 30s, and 72°C for 45s, then a final extension cycle of 72°C for 4 minutes. DNA sequencing was performed with the Big Dye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems Inc.) in accordance with the manufacturer’s instructions. The methylation rate was calculated as follows: methylation rate (%) = X÷(X+Y) x 100 (X: the number of methylated molecules, Y: the number of non-methylated molecules).
| Primer | |
|---|---|
| AR Methylation Forward | 5’-TAGAGGGAAAAAGGGTCGAGTTAGTC-3’ |
| AR Methylation Reverse | 5’-AAAACGTTAACTATCGCTAAAACCG-3’ |
| AR non-Methylation Forward | 5’-TAGAGGGAAAAAGGGTTGAGTTAGTT-3’ |
| AR non-Methylation Reverse | 5’-CAAAAAACATTAACTATCACTAAAACCA-3’ |
Table 1: Primers used in Real-Time MSP.
TaqMan Real-Time PCR
Of the 42 patients in the study, complementary DNA (cDNA) was constructed in 15 patients in order to investigate the AR mRNA expression ratio in the granulosa cells. The isolation of RNA and cDNA preparation of the cells following oocyte retrieval was carried out as previously described [12].
Oligonucleotide primers for TaqMan probes were designed using Primer Express (version 1.0, Perkin-Elmer Applied Biosystems) using the Gene Bank database and based on published sequences of mitochondrial DNA. Human glyceraldehyde-3-phosphate dehydrogenase (GAPDH: Perkin-Elmer Applied Biosystems, Tokyo, Japan) was used as an internal standard. The primers used in TaqMan Real-Time PCR and their expected sizes are as follows: AR-forward: 5’-TGTCGTCTTCGGAAATGTTATGA-3’, AR-Reverse: 5’-TTCCTCCTGTAGTTTCAGATTACCAA-3’, AR-TaqMan: 5’-FAMACTCTGGGAGCCCGGA- MGB-3’. Quantitative analysis of AR mRNA levels was performed in a 20 μl reaction containing 1 x TaqMan Universal PCR Master Mix (Perkin-Elmer Applied Biosystems, Tokyo, Japan) and 200 nM for each forward and reverse primer corresponding to 100 nM of TaqMan probe (Perkin-Elmer Applied Biosystems, Tokyo, Japan). PCR conditions were 95o C for 10 minutes, followed by 60o C for 1 minute, for 40 cycles on OneStep realtime PCR (Perkin-Elmer Applied Biosystems, Tokyo, Japan). The relative AR mRNA expression ratio to GAPDH was analyzed using the ΔΔCt method [12].
Statistical analysis
All experiments were performed in duplicate. Statistical calculations were performed using Stat View statistical software (SAS Institute Inc., Cary, NC, USA). The total sample size and actual power were calculated to be 42 (18 + 24) and 0.79, respectively, with α-err = 0.05, β-err = 0.21, and an effect size d = 0.8.
Data followed a normal distribution and was statistically analyzed using one-way analysis of variance. The comparison between the two nonparametric groups was performed using the nonparametric Mann–Whitney U test or the χ2 test. The Pearson correlation coefficient was analyzed in normally distributed data, and differences were considered to be statistically significant at p < 0.05. Furthermore, a forward stepwise multivariate regression analysis was performed in order to determine the factors associated with the methylation rate of the AR promoter region of the granulosa cells using JMP® 11 (SAS Institute Inc., Cary, NC, USA). A value of p < 0.05 was considered statistically significant.
Results
Index stimulated ART cycles
Table 2 depicts the demographic data and clinical data from the index stimulated ART cycle. Mean age and body mass index of patients with endometriosis showed no statistical difference compared with the control group. Serum level of FSH, luteinizing hormone (LH), estradiol (E2), progesterone (P4), Anti-Müllerian hormone (AMH), total dose of human menopausal gonadotrophin (hMG) fertilization rate, high-quality embryo rate, and pregnancy rate were comparable between the two groups; however, mean AFC and number of retrieved oocytes in the endometriosis group were significantly lower than the same two outcomes in the control group (p < 0.05).
| Control (N=24) |
Endometriosis (N=18) |
p | |
|---|---|---|---|
| Age | 36.54±4.30 | 38.2±3.62 | 0.22 |
| BMI (Kg/m2) | 21.12±2.50 | 22.88±4.09 | 0.42 |
| FSH level(mIU/ml) | 6.92±2.66 | 8.92±3.53 | 0.17 |
| LH level(mIU/ml) | 3.36±1.99 | 3.90±3.29 | 0.98 |
| Estradiol level (pg/dl) | 3291.28±2641.86 | 2150.70±1202.37 | 0.39 |
| Progesterone level (ng/dl) | 0.82±0.43 | 0.55±0.11 | 0.20 |
| Total dose of hMG (IU) | 2206.82±778.70 | 2353.85±678.14 | 0.28 |
| AMH (ng/ml) | 2.27±1.64 | 1.82±2.62 | 0.19 |
| Antral follicle count | 15.62±8.73 | 8.91±3.59 | 0.04 |
| Number of mature follicles (>18mm) | 8.32±4.12 | 3.57±2.81 | 0.03 |
| Number of oocyte | 9.42± 5.42 | 4.81±3.37 | 0.006 |
| Number of embryo | 5.67±5.14 | 3.00±2.65 | 0.08 |
| Fertilization rate(%) | 56.90 | 59.75 | 0.63 |
| Grade Ⅰ and Ⅱ embryos rate(%) | 25.65 | 34.39 | 0.59 |
| Pregnancy rate (%) | 25.0 | 16.67 | 0.29 |
Table 2: Patients’ characteristics.
Outcome of Real-time MSP, bisulfite genomic sequences and TaqMan Real- Time PCR
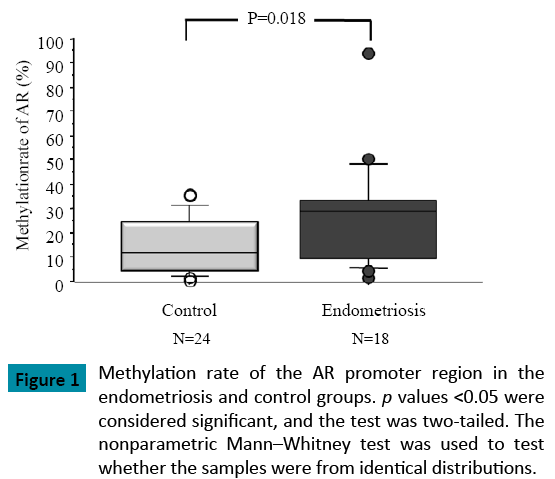
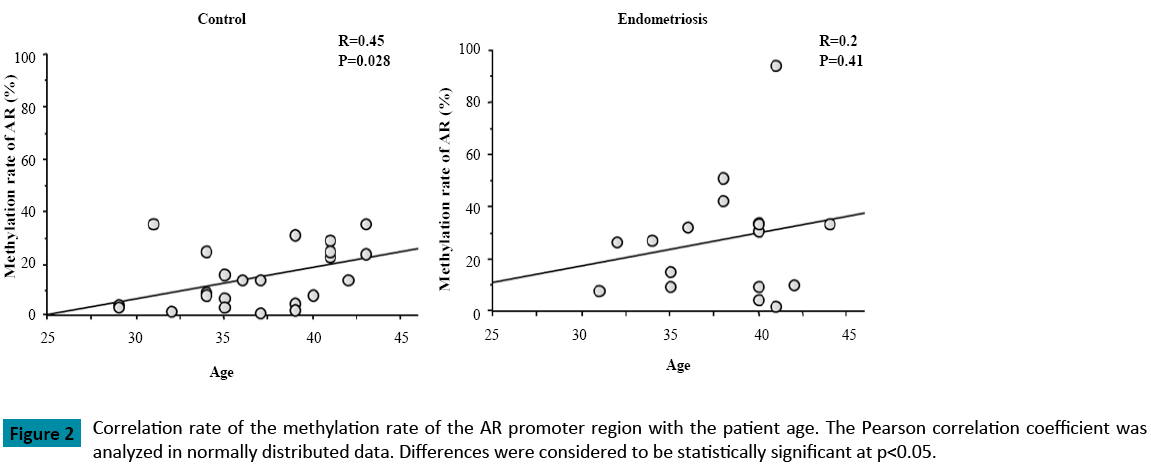
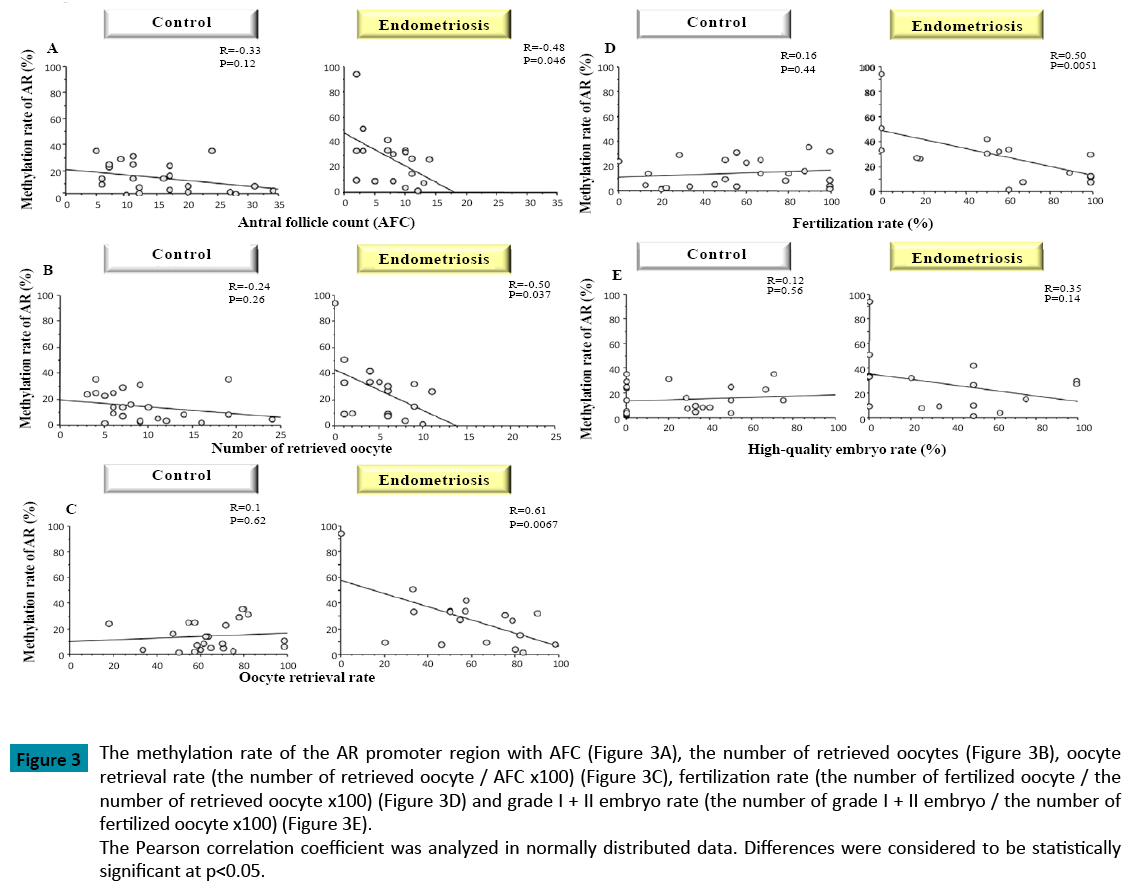
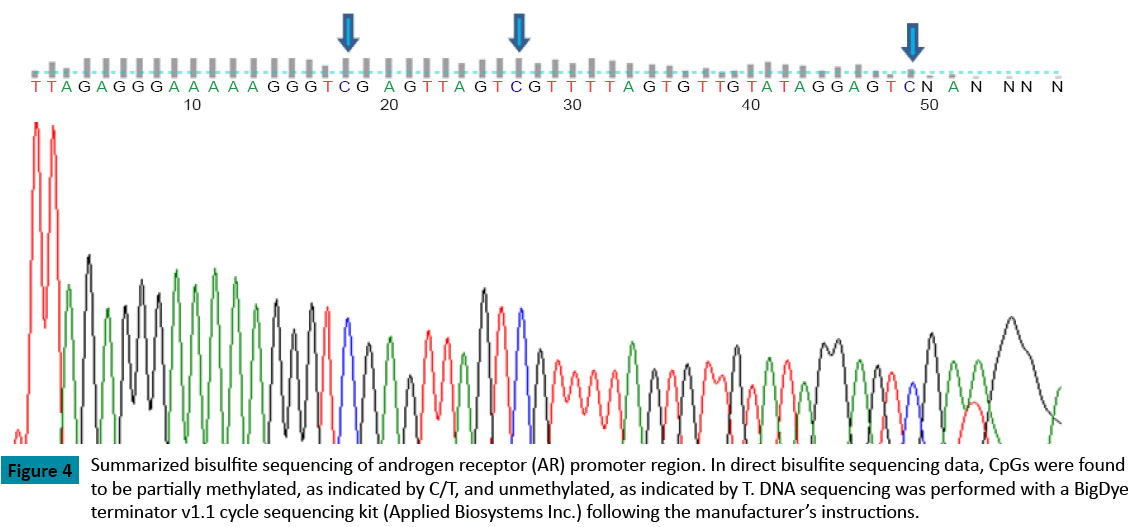
When compared with the control group (p = 0.018), the methylation rate of the AR promoter region was significantly higher in patients with endometriosis (Figure 1). As well, the methylation rate of the AR promoter region was positively correlated with patient age in both groups (Control: R=0.45, Endometriosis 0.2) (Figure 2). In patients with endometriosis, the methylation rate of the AR promoter region was negatively correlated with AFC (Figure 3A), the number of retrieved oocytes (Figure 3B), the rate of retrieval of oocytes (the number of retrieved oocyte / AFC × 100) (Figure 3C), and fertilization rate (the number of fertilized oocyte / the number of retrieved oocyte × 100) (Figure 3D). However, it was not correlated with grade I + II embryo rate (the number of grade I + II embryo / the number of fertilized oocyte × 100) (Figure 3E). Unlike patients with endometriosis, the methylation rate of the AR promoter region in the control group showed no correlation with these factors. Furthermore, direct PCR sequencing data of the AR promoter region showed that CpGs were methylated in all specimens examined (Figure 4).
Figure 3: The methylation rate of the AR promoter region with AFC (Figure 3A), the number of retrieved oocytes (Figure 3B), oocyte
retrieval rate (the number of retrieved oocyte / AFC x100) (Figure 3C), fertilization rate (the number of fertilized oocyte / the
number of retrieved oocyte x100) (Figure 3D) and grade I + II embryo rate (the number of grade I + II embryo / the number of
fertilized oocyte x100) (Figure 3E).
The Pearson correlation coefficient was analyzed in normally distributed data. Differences were considered to be statistically
significant at p<0.05.
Figure 4: Summarized bisulfite sequencing of androgen receptor (AR) promoter region. In direct bisulfite sequencing data, CpGs were found to be partially methylated, as indicated by C/T, and unmethylated, as indicated by T. DNA sequencing was performed with a BigDye terminator v1.1 cycle sequencing kit (Applied Biosystems Inc.) following the manufacturer’s instructions.
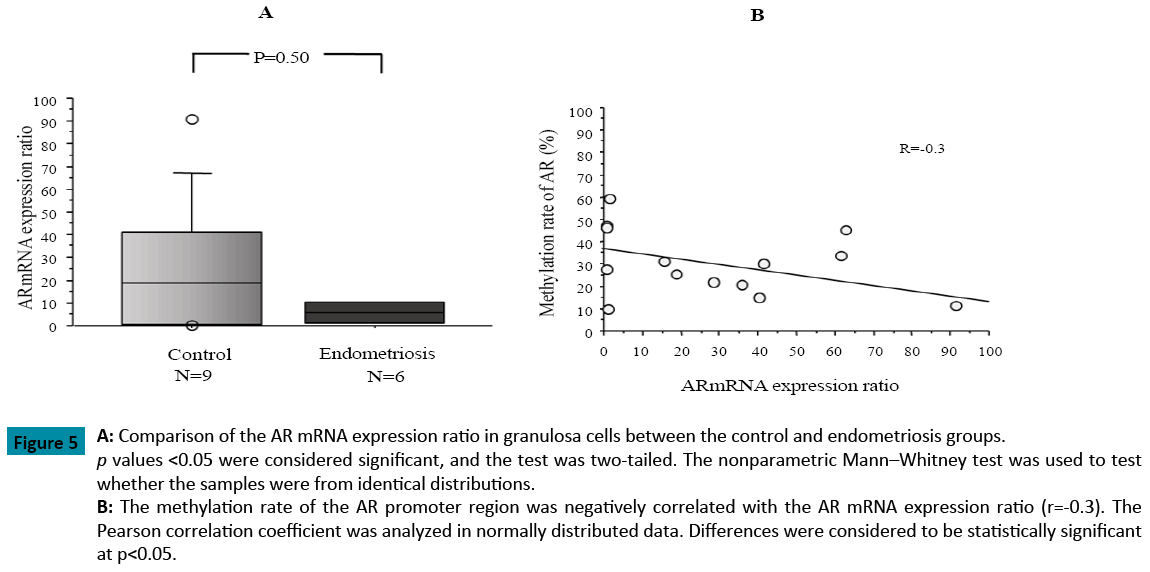
Moreover, TaqMan Real-Time PCR indicated that the AR mRNA expression ratio in the granulosa cells from patients with endometriosis was lower than that in the control group. Additionally, the AR mRNA expression ratio was negatively correlated with the AR methylation ratio in the 15 patients examined (Figure 5).
Figure 5: A: Comparison of the AR mRNA expression ratio in granulosa cells between the control and endometriosis groups. p values <0.05 were considered significant, and the test was two-tailed. The nonparametric Mann–Whitney test was used to test
whether the samples were from identical distributions.
B: The methylation rate of the AR promoter region was negatively correlated with the AR mRNA expression ratio (r=-0.3). The
Pearson correlation coefficient was analyzed in normally distributed data. Differences were considered to be statistically significant
at p<0.05.
Multiple analyses of variance
Forward stepwise multivariate regression analysis revealed that age and endometriosis were significantly and independently associated with the methylation rate of the AR promoter region (Table 3).
| Confouding variables | F value | P value |
|---|---|---|
| Presence of endometriosis | 5.09 | 0.03 |
| Age | 13.98 | 0.0022 |
| BMI(kg/m2) | 0.03 | 0.86 |
Table 3: Multivariate analysis of variance to evaluate contributors to the methylation rate of AR.
Discussion
The present study evaluated human granulosa cell material from mature follicles retrieved from ovaries under COH. The present study showed that: 1) the methylation rate of the AR promoter region was significantly higher in patients with moderate to severe endometriosis when compared with the control group; 2) the methylation rate of the AR promoter region correlated negatively with AFC, number of retrieved oocytes, and rate of retrieval of oocytes; and 3) the AR methylation rate of the AR promoter region was positively correlated with patient age in both groups.
These findings support the concept that moderate to severe endometriosis is associated with the methylation of the AR promoter region, thus leading to poor follicular development.
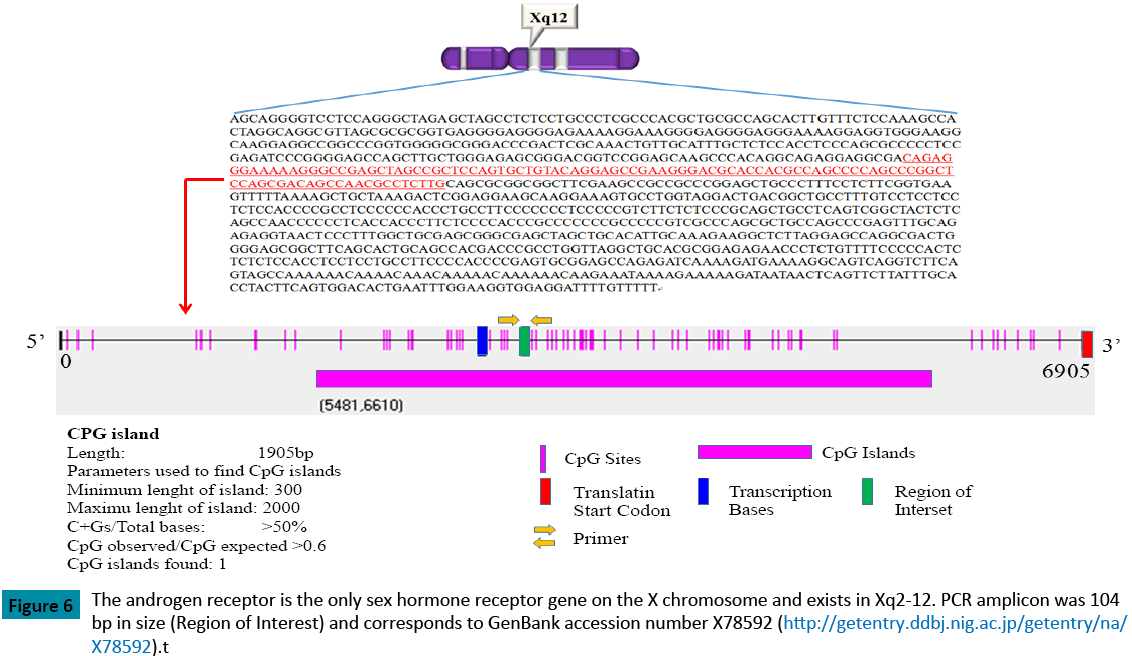
Granulosa cells are the main site of AR expression in human ovaries [16,17]. The expression is highest in early and pre-antral follicles and gradually decreases as the follicles mature [6,7,11]. The AR protein is a key mediator in the enhancement of FSHstimulated follicular differentiation [6-8,17], whereas androgen has been thought to inhibit follicular differentiation and induce follicular atresia [9,10]. AR is present in granulosa cells at almost every stage, particularly in the mid and late-follicular phase [11,18], and AR-mediated androgen signaling is considered to play an important role in the female reproductive system, such as in follicular development and luteinization [11]. Recently, it has been reported that AR knockout mice exhibit a fewer number of antral follicles and retrieved oocytes at 16 weeks of puberty [19]. The androgen receptor is also the only sex hormone receptor gene on the X chromosome and exists in Xq2-12. This gene is more than 90 kb long and codes for a protein that has 3 major functional domains: the N-terminal domain, the DNA binding domain, and the androgen binding domain (Figure 6). The AR gene contains 2 highly polymorphic trinucleotide repeat segments that encode polyglutamine and polyglycine tracts in a protein whose length and methylation patterns affect both AR expression and function. An increase in androgen receptors, induced by estrogen, is perceived as one of the biological phenomena related to the estrogen-induced growth of uterine endometrium. Moreover, Cardenas et al. reported that androgens may be responsible for the regulation of the amount of FSH-receptor mRNA in ovarian follicles [20]. Female AR–/–mice develop a premature ovarian failure (POF) phenotype, and the number of healthy follicles in the AR–/–ovary gradually declines at 3 weeks of age with a marked increase of atretic follicles. By 40 weeks, these AR–/–mice become infertile, with no follicles detectable in the ovary [18]. These findings clearly indicate that AR-mediated androgen signaling is indispensable for the maintenance of folliculogenesis and implicate impaired androgen signaling as a potential cause of POF [18]. Moreover, in cases of prostate cancer, Takahashi et al. reported that AR methylation may represent a phenotype important in the development of hormone independence in a subset of advanced prostate cancers in which AR expression is not found [21]. Jarrard et al., moreover, reported that the methylation of the androgenreceptor- promoter CpG Island is associated with the loss of androgen receptor expression in prostate cancer cells [22]. In the present study, we were unable to analyze the AR mRNA expression ratio in all cases because the sample quantity was too small to allow for the extraction of DNA and mRNA. Factors that are related with the methylation of CpG islands involve aging, chronic inflammation, and viral infection; however, in this study, we were also unable to determine the possible mechanism of a higher methylation rate of the AR promoter region in endometriosis.
Figure 6: The androgen receptor is the only sex hormone receptor gene on the X chromosome and exists in Xq2-12. PCR amplicon was 104 bp in size (Region of Interest) and corresponds to GenBank accession number X78592 (https://getentry.ddbj.nig.ac.jp/getentry/na/X78592).t
There are some limitations to this study. First, the enrolled endometriosis group undertook laparoscopic surgery. This fact raises the possibility that the methylation changes were not specific to endometriosis and, instead, reflect that methylation was caused by the prior laparoscopic treatment itself. Second, the limited number of samples prevented the examination of epigenetic alterations at different stage of endometriosis. However, this study indicates that AR methylation ratio is negatively correlated with AR mRNA expression and that the AR mRNA expression in granulosa cells from patients with endometriosis is lower than that in granulosa cells from patients without the disease. It can, therefore, be hypothesized that AR genes from patients with moderate to severe endometriosis are more methylated and, therefore, less active than in patients of the same age without severe endometriosis.
Here, we have investigated for the first time the relationship between infertility complicated due to moderate to severe endometriosis and the methylation rate of the AR promoter region in granulosa cells.
In summary, our results need to be confirmed in an unselected population, and further studies are needed in order to clarify the influence of endometriosis on not only the methylation rate of the AR promoter region but also on the function of AR in granulosa cells. The present study, however, indicates that a higher methylation rate of the AR promoter region leads to lower mean AFC and number of retrieved oocytes in patients with moderate to severe endometriosis.
Acknowledgement
The authors would like to thank the laboratory technicians Junko Hayashi and Kumiko Sato of the Department of Obstetrics and Gynecology for their assistance and skillful work.
Disclosure
All authors declare that potential conflicts of interest do not exist in this study.
References
- Tummon IS, Maclin VM, Radwanska E, Binor Z, Dmowski WP (1988) Occult ovulatory dysfunction in women with minimal endometriosis or unexplained infertility. FertilSteril 50: 716-720.
- Simón C, Gutiérrez A, Vidal A, de los Santos MJ, et al. (1994) Outcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donation. Hum Reprod 9: 725-729.
- Wardle PG, Foster PA, Mitchell JD, McLaughlin EA, Sykes JA, et al. (1986) Endometriosis and IVF: effect of prior therapy. Lancet 1: 276-277.
- Trukhacheva E, Lin Z, Reierstad S, Cheng YH, Milad M, et al. (2009) Estrogen receptor (ER) beta regulates ERalpha expression in stromal cells derived from ovarian endometriosis. J ClinEndocrinolMetab 94: 615-622.
- Bulun SE (2009) Endometriosis. N Engl J Med 360: 268-279.
- Hillier SG (1985) Sex steroid metabolism and follicular development in the ovary. Oxf Rev ReprodBiol 7: 168-222.
- Daniel SAJ, Armstrong DT. (1986) Androgens in the ovarian microenvironment. SeminReprodEndocr4: 89-98.
- Gore-Langton RE, Armstrong DT. (1988) Follicular steroidogenesis and its control. PhysiolReprod331-385.
- Tsafriri A, Braw RH (1984) Experimental approaches to atresia in mammals. Oxf Rev ReprodBiol 6: 226-265.
- Hsueh AJ, Billig H, Tsafriri A (1994) Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev 15: 707-724.
- Horie K, Takakura K, Fujiwara H, Suginami H, Liao S, et al. (1992) Immunohistochemical localization of androgen receptor in the human ovary throughout the menstrual cycle in relation to oestrogen estrogen and progesterone receptor expression. Hum Reprod 7: 184-190.
- Hayashi M, Yamashita Y, Hayashi A, Yoshida Y, Kawabe S, et al.(2012) Expression and Epigenetic Change of the AR and FSHR Genes in the Granulosa Cells of Endometriosis Patients. Genetics & Epigenetics4:1-8
- Yoshiki Y, Ueda M, Takehara M, Terai Y, Hang YC, et al. (2002) Influence of severe endometriosis on gene expression of vascular endothelial growth factor and interleukin-6 in granulosa cells under controlled ovarian stimulation for IVF-ET. FertilSteril78:865-871.
- Toya M, Saito H, Ohta N, Saito T, Kaneko T, et al. (2000) Moderate and severe endometriosis is associated with alterations in the cell cycle of granulosa cells in patients undergoing in vitro fertilization and embryo transfer. FertilSteril 73: 344-350.
- Hernández HG, Tse MY, Pang SC, Arboleda H, Forero DA (2013) Optimizing methodologies for PCR-based DNA methylation analysis. Biotechniques 55: 181-197.
- Tetsuka M, Hiller SG. (1997) Differential Regulation of Aromatase and Androgen Receptor in Granulosa Cells. J. Steroid BiochemMolec. Biol61: 23-3239.
- Tetsuka M, Whitelaw PF, Bremner WJ, Millar MR, Smyth CD, et al. (1995) Developmental regulation of androgen receptor in rat ovary. J Endocrinol 145: 535-543.
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, et al. (2006) Premature ovarian failure in androgen receptor-deficient mice. ProcNatlAcadSci USA 103: 224-229.
- Hu YC, Wang PH, Yeh S, Wang RS, Xie C, et al. (2004) Subfertility and defective folliculogenesis in female mice lacking androgen receptor. ProcNatlAcadSci USA 101: 11209-11214.
- Cárdenas H, Herrick JR, Pope WF (2002) Increased ovulation rate in gilts treated with dihydrotestosterone. Reproduction 123: 527-533.
- Takahashi S, Inaguma S, Sakakibara M, Cho YM, Suzuki S, et al. (2002) DNA methylation in the androgen receptor gene promoter region in rat prostate cancers. Prostate 52: 82-88.
- Jarrard DF, Kinoshita H, Shi Y, Sandefur C, Hoff D, et al. (1998) Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res 58: 5310-5314.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences