Impact of Crude Oil-induced Oxidative Stress and Lipid Peroxidation on Spermatogenesis: The Anti-Oxidative Role of Ageratum conyzoides
Ita SO*, Effiong EF, Okon MS, Robert AS and Francis UE
Ita SO*, Effiong EF, Okon MS, Robert AS and Francis UE
Department of Physiology, Faculty of Basic Medical Sciences, University of Uyo, Nigeria
- *Corresponding Author:
- Ita Sunday Otu
Faculty of Basic Medical Sciences
Department of Physiology
University of Uyo, Nigeria
Tel: 2348033890830
E-mail: uloro2003@yahoo.com
Received Date: December 11, 2017; Accepted Date: January 17, 2018; Published Date: January 24, 2018
Citation: Ita SO, Effiong EF, Okon MS, Robert AS, Francis UE. (2018) Impact of Crude Oil-induced Oxidative Stress and Lipid Peroxidation on Spermatogenesis: The Anti-Oxidative Role of Ageratum conyzoides. J Reproductive Endocrinol & Infert. Vol.3 No.1:26.
Abstract
The ameliorative potentials of Ageratum conyzoides against crude oil-induced testicular oxidative stress were investigated. Forty male wistar rats (119-128 g body weight) were divided into eight groups of five rats each. The rats in group I served as the control and were oral gavaged 3 mL/kg of normal saline; groups II-IV gavaged 374.17 mg/kg, 748.33 mg/kg and 1122.50 mg/kg body weight of the extract of A. conyzoides respectively, which were calculated as 10%, 20% and 30% of the LD50 (3741.66 mg/kg) respectively. Group V was gavaged 3 mL/kg body weight of Nigerian Bonnylight crude oil (NBLCO) being 20% of the lethal dose of 14.14 ml/kg. Groups VI-VIII animals were administered the aforementioned doses of the extract of A. conyzoides, and 3 mL/kg body weight of NBLCO respectively for 31 days according to animal’s most recent body weight. The results showed that NBLCO significantly reduce super oxide dismutase (SOD) with respect to groups I-IV (P<0.05), but co-administration of A. conyzoides extract to groups VIVIII significantly raise SOD level (p<0.05). On the other hand, NBLCO significantly increase catalase and malondialdehyde levels with respect to groups I-IV (p<0.05); the co-administration of A. conyzoides with NBLCO significantly reduced the aforementioned parameters dose-dependently when compared with NBLCO group (p<0.05). It is concluded that NBLCO administration precipitated testicular oxidative stress which co-administration of Ageratum conyzoides has confirmed in this study to show some therapeutic potentials to ameliorate this pathology.
Keywords
Ageratum conyzoides; Rats; Crude oil; Oxidative stress; Lipid peroxidation; Infertility
Introduction
Human environment has been inundated with many kinds of environmental insults with the tendency of causing injuries to the body by induction of oxidative stress, in most cases resulting in pathologies. Exposures to environmental pollutants affect reproductive function of the male fertility and reproductive health [1]. The high sensitivity of the male reproductive system to high pollutant chemicals and physical agents which are commonly generated and readily present in the environment in diverse forms makes it highly susceptible. Suffice it to state here that infertility, one of the problems of human society is defined to be the inability of a sexually active, non contracepting couple to achieve spontaneous pregnancy in one year [2].
The available data in literature have proposed oxidative stress and lipid peroxidation as the mechanism mediating the hazardous effects of toxicants in the body. Oxidative stress results from the imbalance between the pro-oxidant radicals in excess of the antioxidant capacity of the stressed tissue. Testicular oxidative stress can lead to sperm DNA damage, sperm damage, abnormal sperm morphology, sperm function, and may ultimately results in male infertility. It is also important to note that oxidation of lipids is a critical factor in the pathogenesis of several disease conditions. Excessive generation of ROS can be instigated by uncontrollable exposure to environmental toxicants as well as the physical conditions to affect male fertility. The peroxidation and oxidation of many cell organic molecules including lipids, proteins, carbohydrates, and nucleic acids remained the primary sources of oxidative stress in the body. This is even alarming where there is unnecessary exposure to lipophilic toxicants. Most environmental toxicants like crude petroleum are highly lipophilic in nature and readily accumulate in fatty tissue and such accumulation particularly around the scrotum and testes might disrupt the normal reproductive hormone profile involved in spermatogenesis where they act as endocrine disruptors [3].
ROS in the body in physiological condition is reasonably produced by macrophages, neutrophils and spermatozoa [4] and other cell types under pathologic conditions. Superoxide anions are largely generated as a result of redox reactions within the mitochondria, but in most situations superoxide is quickly converted to hydrogen peroxide by the enzyme superoxide dismutase (SOD) [5,6]. Hydrogen peroxide so produced can be damaging inside the cell because they can cause the covalent cross-linking of a variety of biological molecules as well as the propagation of other free radicals through more complex reactions. The hydrogen peroxide is therefore rendered harmless by reduction reaction involving the enzyme catalase to yield oxygen and water [5,7]. The presence of free radicals and ROS including the superoxide anion and hydrogen peroxide within physiological limit can induce positive changes in sperm function like hyperactivation, capacitation and acrosome reaction. However, excessive production of ROS can be detrimental to sperm and may lead to male infertility because sperm plasma membrane is particularly susceptible to peroxidative damage. The lipid peroxidation destroys the structure of lipid matrix in the membranes of spermatozoa, which most often result in loss of motility and defective membrane integrity [8]. In an experiment such as this, evaluation of the level of malondialdehyde (MDA) generated will suffice the assessment of lipid peroxidation. Testicular oxidative stress can therefore instigate germinal cell death and ultimately hypospermatogenesis. Thus, testicular oxidative stress appears to be a major underlying male factor in infertility. Therefore, a search for botanical source with strong antioxidant bioavailability with tendency to reduce testicular oxidative stress and lipid peroxidation could offer better antioxidant therapies for hypospermatogenesis [9-12].
Increasing evidence indicates that crude oil causes cellular injuries by induction of oxidative stress through the formation of ROS and inhibiting the efficiency of the antioxidant defense system. Studies have shown that ROS-mediated damage to sperm has contributed considerably to the pathology in unselected infertile patients from about 30% to 80% [13]. In the light of this, the ameliorative potentials of Ageratum conyzoides against crude oil-induced testicular oxidative stress and lipid peroxidation in male wistar rats were investigated.
Materials and Methods
The crude petroleum used in this study was obtained from the Exxon Mobil laboratory, Ibeno, Nigeria.
The whole plant was obtained from the Botanical farm of the Department of Pharmacognosy and Natural Medicine, University of Uyo, Nigeria. Specimen of the leaves was authenticated by an expert in the Department of Botany and Ecological Studies, University of Uyo. A voucher specimen (UUH 3517) was deposited at the Herbarium.
Preparation of leave extract
The leaves of A. conyzoides were rinsed with distilled water and dried under shade. The dried leaves were ground into powder with an electric blender. Four hundred grams of the blended leaves sample was macerated in 700 mL 70% ethanol agitated for 10 minutes with an electric blender and left overnight in a refrigerator at 4°C. The mixture was filtered with a cheese cloth and the filtrate obtained concentrated under reduced pressure using a rotary evaporator (at 37°C) to about 10% of its original volume. The concentrate was then allowed in a water bath at 37°C for complete evaporation to dryness yielding 40.64 g (10.16%) of the extract.
Acute toxicity test
Acute toxicity study (LD50) was estimated using Lorke’s method [14]. A total of 25 mice weighing between 15 and 22 g were divided into five groups with five mice per group. Mice in the five groups were respectively administered 3000 mg/kg, 3500 mg/kg, 4000 mg/kg, 4500 mg/kg and 5000 mg/kg of body weight intraperitoneally. All experimental animals were observed for physical signs of toxicity such as gasping, palpitation, writhing, decreased respiratory rate, body limb and death after 24 hours.
The median lethal dose of Ageratum conyzoides was calculated as geometrical means of the maximum (most tolerable) dose producing 0% mortality (a) and the minimum (least tolerable) dose producing 100% mortality (b) using the formula:
LD50=√ab
LD50=√3500 x 4000
=3741.66 mg/kg.
The acute toxicity test for the NBLCO also involved 25 mice weighing between 15 and 22 g were divided into five groups with five mice per group. Mice in the five groups were administered intraperitoneally 10 mL/kg, 15 mL/kg, 20 mL/kg, 25 mL/kg and 30 mL/kg of body weight respectively.
LD50=√10 × 20
=14.14 mL/kg.
Experimental animals
Forty male wistar rats (119-128 g body weight) were obtained from the Animal House of the Faculty of Basic Medical Sciences University of Uyo, Nigeria and were kept in a well-ventilated section of the Animal House. They were allowed access to feed (Chow: vital feeds, Grand Cereals Ltd, Jos) and water ad libitum. The animals were kept in separate experimental room and allowed to acclimatize for a period of one week before commencement of studies.
Experimental design and treatment of animals
A total of forty male wistar rats (119-128 g body weight) were randomly divided into eight groups (I-VIII) of five rats each. The rats in group I served as the control and were oral gavaged 3 mL/kg of normal saline; groups II-IV gavaged 374.17 mg/kg, 748.33 mg/kg and 1122.50 mg/kg body weight of the extract of A. conyzoides as low, medium and high doses, respectively, which were calculated as 10%, 20% and 30% of the LD50 (3741.66 mg/kg) respectively. Group V was gavaged 3 mL/kg body weight of NBLCO; this dose was calculated as 20% of the lethal dose of 14.14 mL/kg. Groups VI-VIII animals were administered the aforementioned doses of the extract of A. conyzoides and 3 mL/ kg body weight of NBLCO respectively. In all cases, doses were applied daily for 31 days according to animal’s most recent body weight. The experimental procedures involving the animals and their care were conducted in conformity with the approved guidelines by the Research and Ethical Committee of the Faculty of Basic Medical Sciences, University of Uyo, Nigeria.
Collection of blood sample for analysis
After the thirty one (31) days of administration, the rats were anaesthetized with chloroform soaked in swap of cotton wool in a killing chamber. Blood was collected by cardiac puncture with a 5 mL sterile syringe and needle. The total volume of blood collected was 3.5 mL, which was transferred into plain sample bottles. This was allowed to stand for 2 hours to clot after which the serum was separated by centrifugation (RM-12 micro centrifuge, REMI, England) at 3000 rpm for 10 minutes at 25°C. The serum obtained was stored at -20°C until required for analysis.
Super oxidase dismutase assay (SOD)
Serum superoxide dismutase (SOD), catalase (CAT), and malondialdehyde (MDA) were assayed using standard laboratory methods [15,16].
Statistical analysis
Data were expressed as the mean ± standard error of the mean. Statistical analysis was carried out using window SPSS package (SPSS 22.00 version). Data were analyzed using one way analysis of variance (ANOVA), results obtained were further subjected to test for least significant difference (LSD). Values of P<0.05 were considered significant.
Results
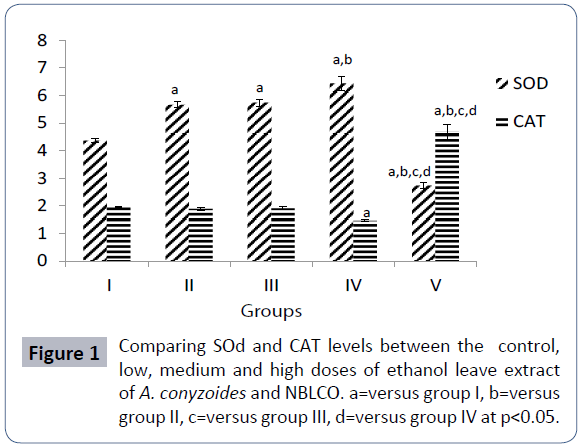
The results of superoxide dismutase (SOD) activity obtained following the administration of Nigerian Bonny light crude oil (NBLCO) and or administration of leaf extracts of Ageratum conyzoides for groups I, II, III, IV and V are 4.36 ± 0.07, 5.66 ± 0.11, 5.74 ± 0.12, 6.44 ± 0.26 and 2.73 ± 0.11 (mg/dL), respectively.
The results showed that administration of ethanolic leaf extracts of Ageratum conyzoides significantly increased SOD activity with respect to the control group (p<0.05) in a dose-dependent fashion. But administration of NBLCO to group V rats significantly reduced SOD with respect to the control and A. conyzoides treated groups (p<0.05) as shown in Figure 1.
The results of catalase (CAT) activity obtained following the administration of Nigerian Bonny light crude oil (NBLCO) and coadministration of leaf extracts of Ageratum conyzoides for groups I, II, III, IV and V are 1.96 ± 0.02, 1.89 ± 0.06, 1.91 ± 0.05, 1.46 ± 0.04 and 4.68 ± 0.27 (mg/dL), respectively. The administration of low and medium doses of ethanolic leaf extracts of Ageratum conyzoides did not alter catalase activity significantly with respect to the control group but the high dose caused a significant reduction (p<0.05), while administration of NBLCO significantly increased CAT activity higher than the control A. conyzoides groups (p<0.05) as shown in Figure 1.
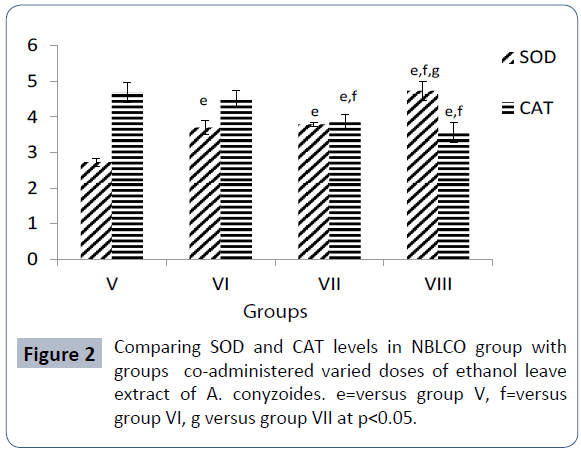
The mean values of SOD activity obtained for groups V, VI, VII, and VIII are 2.73 ± 0.11, 3.70 ± 0.19, 3.79 ± 0.05 and 4.73 ± 0.26 respectively. The results as presented in Figure 2 showed that co-administration of ethanolic leaf extracts of A. conyzoides to groups VI-VIII significantly increased SOD activity in a dosedependent fashion with respect to group V rats that were administered only NBLCO (p<0.05).
The results of CAT activity obtained for groups V, VI, VII and VIII are 4.68 ± 0.27, 4.52 ± 0.22, 3.86 ± 0.21 and 3.56 ± 0.29 respectively. The results showed that co-administration of ethanolic leaf extracts of A. conyzoides to groups VI-VIII increased CAT activity in a dose-dependent fashion with medium and high dose groups differing from group V significantly (p<0.05) as presented in Figure 2.
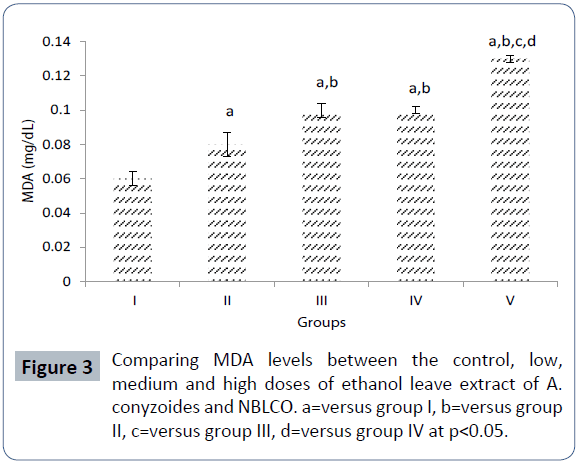
The results of male (MDA) level obtained following the administration of Nigerian Bonny light crude oil (NBLCO) and or administration of leaf extracts of Ageratum conyzoides for groups I, II, III, IV and V are 0.06 ± 0.004, 0.08 ± 0.007, 0.10 ± 0.004, 0.10 ± 0.002 and 0.13 ± 0.002 (mg/dL), respectively.
The results showed that administration of ethanolic leaf extracts of Ageratum conyzoides significantly increased MDA level activity with respect to the control group (p<0.05). And administration of NBLCO to group V rats significantly increased MDA with respect to the control and A. conyzoides treated groups (p<0.05) as shown in Figure 3.
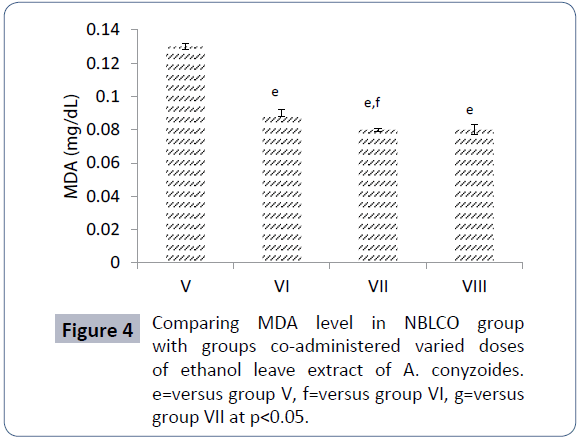
The results of MDA level obtained for groups V, VI, VII, and VIII are 0.13 ± 0.002, 0.09 ± 0.002, 0.08 ± 0.001 and 0.08 ± 0.003 respectively. The results as presented in Figure 4 showed that co-administration of ethanolic leaf extracts of A. conyzoides to groups VI-VIII significantly reduced MDA level with respect to group V rats that were administered only NBLCO (p<0.05) as shown in Figure 4.
Discussion
The ameliorative potentials of Ageratum conyzoides against crude oil-induced testicular oxidative stress and lipid peroxidation were investigated. Oxidative stress is consequence upon the imbalance between reactive oxygen species (ROS) and antioxidants in the body and has been identified as an important factor that influences male fertility status [17-19].
Increasing evidence indicates that crude oil could induce oxidative stress as well as apoptosis by inducing the formation of ROS and inhibiting the efficiency of the endogenous antioxidant defense systems, this then result in pathology. ROS though essential in physiological limit for sperm capacitation, unrestricted ROS generation can be pathologic affecting particularly the male reproductive health as it play a major role in oxidative stress and its complications, including male infertility. The administration of NBLCO could significantly induce oxidative stress in the testis by decreasing the levels of SOD as reported in the present study. This allows the super-oxidant generation to flourish unabated, this especially in sperm, is harmful because it damages sperm DNA and causes apoptosis in sperm. The hazardous effect of crude petroleum in reducing the number of spermatogenic cells, the diameter of the seminiferous tubules, induction of cell apoptosis and atrophy in the seminiferous tubules, which are indicators for the failure of spermatogenesis is well documented in a literature [20,21].
Administration of crude petroleum and some of its refined products induces oxidative stress in the testis by increasing the formation of free radicals while suppressing the activities of endogenous antioxidant systems particularly the antioxidant enzymes. It has been postulated that increase production of ROS could cause mitochondrial injury to trigger apoptotic process leading to the death of sperm cells and lipid peroxidation in both, germ and leydig cells and subsequently a defect in sperm production [22]. This study has revealed that NBLCO significantly instigated lipid peroxidation as demonstrated with the significantly high MDA level recorded and associated testicular cellular injuries to impaired normal processes of spermatogenesis, in agreement with other studies, where increased levels of MDA is reported to be associated with decreased sperm motility [23,24].
It was also observed that the activity of testicular SOD was significantly inhibited by the NBLCO suggestive of an inducedoxidative stress orchestrated by reduced activities of testicular antioxidant enzymes. In some other studies, the association between low testicular testosterone production and conditions such as aging and cryptorchidism is reported to be associated with substantial ROS production and oxidative stress in the testis [25,26]. It is also true that during steroidogenesis ROS is largely produce from mitochondrial respiration and the catalytic reactions of the steroidogenic cytochrome P450 enzymes [27-29]. The NBLCO by inducing lipid peroxidation and ROS generation, if unchecked by the seemingly overwhelmed endogenous antioxidant systems can also damage mitochondrial membranes to escalate apoptotic process and this contribute significantly to sperm death and the inhibition of subsequent steroidogenesis [30]. An ultimately inhibiting spermatogenesis and spermatozoa functions, an inhibited or complete absence of spermatogenesis is indicative of male infertility.
In contrast, the co-administration of the extract of A. conyzoides significantly reversed this trend by restoring the activity of SOD and reducing the level of lipid peroxidation. These observations indicate the spermatogenic cell-protective potentials of A. conyzoides in biological systems and could play a fundamental role in sperm maturation, which could be credited to the rich antioxidant content of the A. conyzoides. The findings of this study are very important as ROS is known to be an independent marker for male factor infertility as it can lead to DNA damage, spermatozoa deformity and damage of plasma membrane integrity in sperm [13]. Antioxidants are very important as they react with and scavenged ROS assisting in preventing and or reducing oxidative stress onset and preserving spermatogenic processes and spermatozoa functions [1].
Conclusion
Testicular oxidative stress and lipid peroxidation by interrupting spermatogenic processes play fundamental roles in male infertility. It is concluded that NBLCO administration precipitated testicular oxidative stress and lipid peroxidation with concomitant disruption in normal spermatogenesis which could cause infertility in male rats. The ethanolic leaf extracts of Ageratum conyzoides has be confirmed in this study to show some therapeutic potentials to ameliorate associated pathologies by arresting the hypospermatogenesis induced by NBLCO via oxidative stress and lipid peroxidation.
References
- Sharma RK, Agarwal A (1996) Role of reactive oxygen species in male infertility. Urology 48:835-850.
- Zegers Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R (2009) The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary on art terminology. Hum Reprod 24:2683-2687.
- Oliva A, Spira A, Multigner L (2001) Contribution of environmental factors to the risk of male infertility. Hum Reprod 16:1768-1776.
- Aitken RJ, Clarkson JS (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fert 81: 459-469.
- Mates JM, Sanchez Jimenez F (1999) Antioxidant enzymes and their implications in pathophysiologic processes. Front Biosci 4: 339-345.
- Cadenas E (2004)Mitochondrial free radical production and cell signaling. Mol Aspects Med 25: 17-26.
- Pryor WA, Houk KN, Foote CS, Fukuto JM, Ignarro LJ, et al.(2006) Free radical biology and medicine: it's a gas, man! Am J Physiol Regul Integr Comp Physiol 291: R491-R511.
- Sonmez M, Turk G, Yuce A (2005) The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male wistar rats. Theriogenology 63:2063-2072.
- Narra VR, Howell RW, Sastry KSR, Rao DV (1993) Vitamin C as a radioprotector against iodine-131 in vivo. J Nucl Med 34: 637-640.
- Gavazza M, Catala A (2003) Melatonin preserves arachidonic and docosapetaenoic acids during ascorbate-FE++ peroxidation of rat testis microsomes and mitochondria. Int J Biochem Cell Biol 35: 359-366.
- Uguralp S, Usta U, Mizrak B (2005) Resveratrol may reduce apoptosis of rat testicular germ cells after experimental testicular torsion. Eur J Pediatr Surg 15: 333-336.
- Kutlubay R, Oguz EO, Can B, Guven MC, Sinik Z, et al. (2007) Protection from testicular damage caused by intraperitoneal aluminium. Int J Toxicol 26: 297-306.
- Agarwal A, Said TM, Bedaiwy MA, Banerjee J, Alvarez JG (2006) Oxidative stress in an assisted reproductive techniques setting. Fertil Steril 86:503-512
- Lorke D(1993) A New Approach to Practical Acute Toxicity Testing. Arch Toxicol 54: 275-287.
- Adewole SO, Ojewole JA (2009) Protective effects of Annona muricata Linn (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med 6: 30-41.
- Ekaluo UB, Ikpeme EV, Udensi O, Markson AA, Madunagu BE, et al. (2010) Effect of aqueous leaf extract of neem (Azadiraclita indica) on the hormonal milieu of male rats. International Journal of Current Research 4: 1-3.
- Aitken RJ, Roman SD (2008) Antioxidant system and oxidative stress in the testes Oxid Med Cell Longev1: 15-24.
- Apostoli P,Catalani S (2001) Metal ions affecting reproduction and development. Met Ions Life Science8:263-703.
- Wright, C, Milne S, Leeson H (2014) Sperm DNA damage caused by oxidative stress: modifiable clinical lifestyle and nutritional factors in male infertility. Reprod Biomed Online 28:684-703.
- Cai L, Chen S, Evans T, Deng DX, Mukherjee K et al. (2000) Apoptotic germ-cell death and testicular damage in experimental diabetes: Prevention by endothelin antagonism. Urol Res28: 342-347.
- Guneli E, Tugyan K, Ozturk H, Gumustekin M, Cilaker S, et al. (2008) Effect of melatonin on testicular damage in streptozotocin-induced diabetes rats. Eur Surg Res 40: 354-360.
- Li M, Liu Z, Zhuan L, Wang T, Guo S, et al. (2013) Effects of apocynin on oxidative stress and expression of apoptosis-related genes in testes of diabetic rats. Mol Med Rep 7: 47-52.
- Agarwal A, Prabakaran SA (2005) Mechanism, measurement, and prevention of oxidative stress in male reproductive physiology.Indian J Exp Biol 43:963-974.
- Kefer JC, Agarwal A, Sabanegh E (2009) Role of antioxidants in the treatment of male infertility. Int J Urol16: 449-457.
- Zirkin BR, Chen H (2000) Regulation of Leydig cell steroidogenic function during aging. Biol Reprod 63: 977-981.
- Chaki SP, Misro MM, Ghosh D, Gautam DK, Srinivas M (2005) Apoptosis and cell removal in the cryptorchid rat testis.Apoptosis 10: 395-405.
- Peltola V, Huhtaniemi I, Metsa-Ketela T,Ahotupa M(1996) Induction of lipid peroxidation during steroidogenesis in the rat testis. Endocrinolog 137: 105-112.
- Hales BD (2002) Testicular macro phage modulation of leydig cell steroidogenesis. J Reprod Immmunol 57: 3-18.
- Hanukoglu I (2006) Antioxidant protective mechanisms against reactive oxygen species (ROS) generated by mitochondrial P450 systems in steroidogenic cells. Drug Metab Rev 38: 171-196.
- Luo L, Chen H, Trush MA, Show MD, Anway MD, et al.(2006) Aging and the Brown Norway rat Leydig cell antioxidant defense system. J Androl 27: 240-247.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences