Impact of Pre-Mixing AMH Serum Samples with Standard Assay Buffer: Ovarian Reserve Estimations and Implications for Clinical IVF Providers
Kevin D Marron, E Scott Sills, Paul L Cummins, Connor Harrity, David J Walsh and Anthony PH Walsh
1Division of Reproductive Endocrinology and Infertility, Sims IVF/ The Sims Institute, Dublin, Ireland
2Zentra Lab Limited, Dublin, Ireland
3Reproductive Research Section, Center for Advanced Genetics, Carlsbad, California, USA
4Faculty of Applied Biotechnology, School of Life Sciences, University of Westminster, London, United Kingdom
5Department of Obstetrics & Gynaecology, School of Medicine, Royal College of Surgeons in Ireland, Dublin, Ireland
- *Corresponding Author:
- Paul L Cummins
Sims IVF/The Sims Institute
Clonskeagh Road
Dublin 14, Ireland
Tel: +353-0-1-208-0710
Fax: +353-0-1-208-0715
E-mail: paul.cummins@sims.ie
Received date: May 19, 2016; Accepted date: May 28, 2016; Published date: June 4, 2016
Citation: Paul L Cummins, et al. Impact of Pre-Mixing AMH Serum Samples with Standard Assay Buffer: Ovarian Reserve Estimations and Implications for Clinical IVF Providers. J Reproductive Endocrinol& Infert. 2016, 1:10. doi: 10.4172/JREI.100010
Copyright: © 2016, Paul L Cummins et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Recognizing the key role of AMH testing in shaping the medication and dosing protocols for IVF patients, our study aimed to characterize two common AMH testing platforms and compare the results from each. Methods: Standard AMH assay (no buffer) measurements were performed on the AMH Beckman Coulter Gen II device as controls, and samples (n=85) were run in parallel on the same equipment using the manufacturer’s buffer pre-mix of bovine serum albumin with ProClin and Sodium azide as preservatives. Standard vs.modified (pre-mixed/ buffered) assay techniques were also compared retrospectively by using archived serum samples (n=214) obtained at our clinic. Results: Comparison of these two AMH measurement protocols revealed a mean increase in reported values of 39% (range -7 to +300%) using the latter technique, compared to standard assay method. Moreover, interassay variance (standard vs. modified AMH protocol) ranged from -7% to +300%. A retrospective analysis of archived samples found the modified assay method yielded median AMH values 41% higher than results reported by standard assay. Conclusion: While AMH has become increasingly utilized to estimate ovarian reserve, remarkable variation in AMH levels can be reported based on assay techniques. When comparing the standard (non-buffered) AMH assay to the pre-mix/buffered AMH assay, this study provides compelling evidence that the latter testing method will return a significantly elevated value. Clinicians should be aware of these differences and interpret serum AMH measurements carefully, particularly AMH data reported by laboratories using different assay methodologies.
Keywords
Assay variation; Buffer; AMH; IVF; Ovarian reserve
Introduction
Anti-Müllerian hormone (AMH) is a dimeric glycoprotein member of the transforming growth factor-β superfamily, produced by granulosa cells of the ovary. AMH is a key regulator of early ovarian follicular growth and cyclic follicular selection [1,2]. Phylogenetically, AMH is characterised by a highly conserved promoter region and plays a crucial functional role in mammalian reproduction [3]. Clinical practice increasingly relies on serum AMH measurements to provide useful estimates for ovarian reserve, as it directly correlates with the size of the primordial follicle cohort and diminishes over time with reproductive senescence [4]. Of note, serum AMH is favoured at some IVF centres as an ovarian reserve test because of its relative constancy throughout the menstrual cycle [5,6] and its tendency to be unchanged despite GnRH-agonist pituitary down-regulation or pregnancy [7,8]. Although these features of AMH have encouraged its measurement before treatment to elucidate diminished reserve associated with ovarian aging [9,10], there has been little research concerning how pre-analytical factors such as sample buffering may influence the results reported to clinicians.
At present, numerous commercial assay platforms are available to IVF clinics for rapid measurement of AMH levels including the Active MIS/AMH ELISA (Cat. # DSL-10-14400) (DSL; Webster, TX, USA) or EIA AMH/MIS ELISA (Cat. # A16507) (IOT, Marseille, France). Of note, the AMH Gen II platform (Cat. # A79765) (Beckman Coulter; Chaska, MN, USA) is the most commonly used methodology available [11]. This particular ELISA assay relies on two specific antibodies directed against a specifically defined region of the AMH molecule (a structural component thought to be less susceptible to proteolysis), as well as a pro-region of AMH which participates in protein folding [12,13]. Due to some controversy regarding the sensitivity and reproducibility of the GEN II assay, the assay manufacturer (Beckman Coulter) acknowledged that their original methodology could result in potentially erroneous AMH values secondary to complement interference [11]. In response, Beckman Coulter modified the GEN II protocol in July 2013 and introduced a new pre-mixing step; this involved premixing sample serum with assay buffer before plating to minimize the effects of complement, while still retaining an accurate AMH estimation. Unfortunately, this modification passed largely unnoticed by IVF clinicians and was scarcely discussed even in the laboratory methods literature. Yet given the gravitas accorded to serum AMH levels in estimating ovarian reserve, results from this assay help configure gonadotropin stimulation regimes for IVF patients. Thus, any modification to the AMH assay which might cause substantial change the AMH level is not a trivial matter.
The distribution and extent to which the several AMH assay protocols are used among laboratories worldwide cannot be known with precision, and only a handful of investigations have directly compared these methods (i.e., with vs. without the pre-mix buffer variable). Indeed, data available on AMH assay techniques have only recently coalesced and thus far is summarized in just two papers [11,14]. These investigators noted that AMH measurements rise significantly when premixed with assay buffer before plating, giving the IVF clinician an inappropriately elevated reading; this leads to a corresponding (incorrect) interpretation of relatively robust ovarian reserve.
With this background, and recognizing the central role AMH testing can play in guiding the counselling and care of IVF patients, our study aimed to characterize two common AMH testing platforms and compare the results from each. We prospectively evaluated AMH results reported with and without pre-mixing of samples using standard buffer, to quantify the effect on AMH levels that may be attributed to buffering alone. In addition, AMH values were measured in archived samples (using the modified buffered technique) and retrospectively compared to age-matched reference levels for AMH derived from the standard (non-buffered) assay method.
Methods
Study site and sample preparation
Samples were obtained from female IVF patients (n=85) who attended a large European fertility clinic (Sims IVF/The Sims Institute, Dublin, Ireland). All patients provided written informed consent to provide specimens for analysis; samples were collected during their reproductive endocrinology consultation at our institution. Each patient provided only one serum sample used for this investigation. Because this study did not record any patient identifiers (i.e., all samples were tabulated anonymously), and phlebotomy encounters necessary for the study were already being routinely performed, the research was deemed exempt from review by our Institutional Ethics Committee. The study site participates in the pilot National External Quality Assessment Scheme (UKNEQAS) for AMH (Edinburgh) with satisfactory performance over >3 yrs. All samples were transported (within ≤ 2 hr of collection by peripheral venepuncture) to a dedicated laboratory physically adjacent to the IVF unit for analysis. Samples were immediately centrifuged and frozen in aliquots at -20°C until analysis (within 7-10 d). After thaw, specimens were each split equally to facilitate parallel measurement of both assays simultaneously.
Standard AMH assay method (no buffer)
These AMH measurements were performed manually on the AMH Gen II assay (Cat. # A79765) (Beckman Coulter) according to manufacturer 2012 guidelines. Calibration, controls, sample collection, and storage parameters were all in compliance with manufacturer’s original recommendations. Samples were thawed 2 hr before analysis and plated in duplicate, the mean of two replicates was reported as the final result for each sample (pmol/l).
Modified AMH assay method (with pre-mix buffer)
Based on 2013 Beckman Coulter Gen II AMH instructions, these AMH measurements were performed manually on the AMH Gen II assay (Cat. # A79765) (Beckman Coulter). In brief, 60 μl of serum was added to 300 μl of assay buffer and incubated in an automated orbital shaker x 10 min (buffer is supplied as two 13 ml vials provided with the GEN II assay kit, consisting of bovine serum albumin, protein, with ProClin and sodium azide as preservatives). Next, 120 μl of this mixture was transferred to the AMH assay plate in duplicate. Calibrators and controls were also subjected to this standard pre-mix modification. The mean of two replicates was reported as the final result for each sample (pmol/l). The CV for both standard and modified AMH assay methods was validated before the study at <9% and <11%, respectively.
Retrospective comparisons
Assay methods were also evaluated by using archived serum samples obtained at our clinic (n=214) and retrospectively tested by the modified (pre-mixed/buffered) assay technique. These values were stratified by patient age and compared to age-matched reference ranges calculated from 2,301 samples established previously with the standard method.
Statistical analysis
For samples which included the pre-mixing step, results were tabulated as a percentage relative to the value of the standard assay which was considered as a baseline value. A paired two tailed t-test was performed for comparisons using SPSS (Microsoft Windows 7). p<0.05 was considered statistically significant.
Results
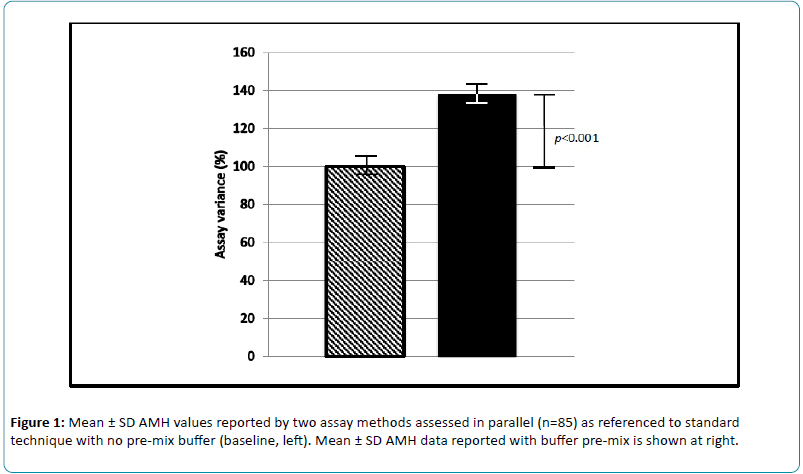
Serum samples were prospectively measured and compared for AMH levels, as measured by standard assay (n=85) and by the modified (pre-mixed) method (n=85). Comparison of these two AMH measurement protocols revealed a mean increase in reported values of 39% (range -7 to +300%) compared to baseline, defined as AMH values from the standard method (Figure 1). Standard deviations for the modified (pre-mixed) and non-buffered assays were ± 23.3 and ± 19.2, respectively. For these samples, inter-assay variance (standard vs. modified AMH protocol) ranged from -7% to +300%.
Retrospective comparisons using banked samples revealed that the modified (pre-mix/ buffered) assay method yielded median AMH values 41% higher than results reported by standard assay (Tables 1 and 2). Mean AMH values with the modified assay were increased by 30.5%, compared to the standard AMH assay method (baseline). Of note, the largest median increase was observed in women aged 31-40 (likely to be a substantial subset of patients attending for IVF), where a +50% rise in AMH levels was observed using the modified (buffered) assay within this group.
| Standard n=2301 | Buffer n=214 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| age(yrs) | n | mean | 25%ile | median | 75%ile | n | mean | 25%ile | median | 75%ile |
| 26-30 | 160 | 30.4 | 13.5 | 23.7 | 38.8 | 4 | 35.7 | 16.1 | 33 | 47.8 |
| 31-35 | 789 | 24.6 | 8.5 | 17.1 | 31 | 84 | 32.4 | 12.4 | 27 | 41.8 |
| 36-40 | 945 | 16.1 | 4.6 | 10.7 | 19.9 | 83 | 20.1 | 5.8 | 15.2 | 28.1 |
| 41-45 | 407 | 9.8 | 2.1 | 5.5 | 11.6 | 43 | 14.5 | 3 | 6.9 | 20.9 |
| Note: All serum AMH values reported as pmol/l. | ||||||||||
Table 1: Comparison of centile reference ranges of serum AMH values reported from standard vs. buffered assay methods, stratified by age.
| Standard | Median | Buffered | Standard | Mean | Buffered | |
|---|---|---|---|---|---|---|
| Age(yrs) | Variance (%) | Variance (%) | ||||
| 26-30 | 23.7 | 39 | 33 | 30.4 | 18 | 35.7 |
| 31-35 | 17.1 | 58 | 27 | 24.6 | 32 | 32.4 |
| 36-40 | 10.7 | 42 | 15.2 | 16.1 | 24 | 20.1 |
| 41-45 | 5.5 | 26 | 6.9 | 9.8 | 48 | 14.5 |
| Aggregate | 41.25% | Aggregate | 30.50% | |||
| Note: All serum AMH values reported as pmol/l. | ||||||
Table 2: Observed variance for serum AMH values using standard and buffered assay, stratified by age.
Discussion
AMH testing is emerging as a fundamental element during the pre-treatment evaluation for the advanced reproductive technologies [15,16]. Thus, AMH assessments must be specific, reproducible, and insensitive to external factors. IVF clinicians who rely on serum AMH measurements to craft the gonadotropin regime used for controlled ovarian hyperstimulation should be able to expect that the data obtained from this routine test will neither increase the IVF patient’s risk for OHSS (ovarian hyperstimulation syndrome) nor increase the risk for cycle cancellation due to poor response. When AMH testing is requested or ordered by the consultant physician in a fertility clinic, there may be a presumption that all assay platforms are the same, thus rendering all AMH results from various laboratories equivalent. The findings from our investigation suggest that this presumption is not valid. Indeed, not knowing which assay technique is being used to measure AMH could bring potentially catastrophic clinical consequences for IVF patients. The present study confronts this issue in two ways, first as a prospective, parallel run of different AMH assay platforms, as well as a larger retrospective run on an even larger series of archived samples for AMH measurement. Interestingly, these data show a consistent (and significant) rise in reported AMH levels when the pre-mix buffer step in included in the laboratory protocol, compared to an older assay process which did not include this pre-mix buffering component.
In addition to investigating two AMH assay techniques prospectively, the current study assessed how these two assay methods could lead to variances in AMH reference ranges using archived samples. In this respect, our work is the first to show that the pre-mix/buffer step is likely to produce the greatest absolute increases in AMH among samples obtained from patients at the extremes of age. In our study population, women age 31-40 yrs produced serum samples where mean AMH serum levels varied by +30.5%, compared to the standard AMH assay method. Such variances have particular relevance for oocyte donors and those of advanced maternal age, where risks for OHSS and cycle cancellation may be improperly estimated due to assay differences described here [17].
Ours is not the first research effort to draw attention to how AMH levels may be different depending on how it is measured [18]. The Beckman Coulter GEN II AMH assay is commonly used both by commercial laboratories serving the general medical community and, increasingly, by reproductive medicine practices. Several researchers had initially observed poor reproducibility and significant variations in AMH values, but were uncertain as to what factors might explain this phenomenon [14,17,19]. By July 2013, the manufacturer had introduced a new sample incubation step in response to these concerns—this dilution buffer step was intended to decrease compliment interference.
Before the manufacturer first released their GEN II assay for AMH, a multicentre analysis was conducted to evaluate assay performance and compare same to the Immunotech (IOT) assay, considered the predecessor to the GEN II assay platform. That important study [20] identified a +40% change in values obtained using the GEN II. To our knowledge, subsequent to the July 2013 introduction of the pre-mixing/ buffer step, the assay manufacturer has published no further data concerning the effects produced by the pre-mix/buffering component in AMH testing. Our investigation presents original, comparative data on the effects of pre-mixing samples with assay buffer prior to plating, and finds that adding the pre-mixing buffer step increased mean AMH serum level values substantially over matched samples run in parallel assay with no buffer step (mean reported AMH level being higher by approximately 38%). These results agree with previous data, where increased AMH values were noted when samples were pre-mixed with assay buffer, compared to results obtained without the pre-mix/buffer component [14]. More recently, AMH levels assessed from four separate control groups found a >100% increase in median pre-mixed AMH levels, compared to the standard method which did not incorporate buffer [11].
Such observations mean that it is no longer sufficient simply to “order an AMH test.” Clinicians interested in AMH must also be aware of which assay type is being used to measure this analyte. Of course, this distinction becomes particularly relevant with serial AMH determinations measured by more than one laboratory, although the crucial question remains: Why does this inter-assay variation exist with respect to serum AMH testing? One intriguing theory [14] suggests that premixing might expose previously masked secondary epitopes on the AMH molecule, and that the extent of this native antigenic “covering” differs between individuals. By pre-mixing with buffer before plating, the AMH molecule may be fully exposed thereby producing increased (albeit consistent and reliable) results. Indeed, Beckman Coulter has claimed endogenous complement can interfere with the AMH assay, and that premixing with the anionic buffer may reduce the effects of complement.
There are some potential limitations in our investigation, and these should be noted. For example, different samples will exhibit altered levels of complement activation depending on numerous physiological factors. Some patients undergoing IVF could have subtle, undiagnosed autoimmune conditions which are known to alter complement C3 and C4 levels in vivo [21,22]. Thus, even a mild autoimmune dysfunction could contribute to some inter-sample variability in AMH values recorded here, independent of assay buffering. The buffer group sample was substantially smaller than the standard nonbuffer group sample, and it would have been preferable to include additional specimens especially from the youngest age classification (26-30 yrs). However, these youngest women would typically constitute the best prognosis IVF patients where limited ovarian reserve would be much less likely. Finally, some laboratories use an automated AMH assay while others rely on a manual method (as in the present study). Values from different testing techniques may not be interchangeable, and such non-uniform and usually unspecified assay methodology may place another variable across any clinical interpretation where all the clinician knowns are that an “AMH test” was completed.
In summary, the present work presents the largest prospective dataset examining reported AMH levels to date, when both standard and pre-mix/buffer methods are compared directly. It is plausible that the manufacturer’s rationale for including the extra buffering/pre-mixing step does indeed improve assay reproducibility by attenuating sample variation. Laboratories not utilizing the pre-mixing/ buffering step are at risk for reporting erroneously low and excessively unstable AMH levels, compared to facilities where the recommended buffering step has been properly incorporated. While the pre-mixing/buffering probably does diminish inter-sample variation, the values obtained from the pre-mix/buffer protocol will require a recalibration of previous age-based AMH nomograms. In any case, we suggest that laboratories utilizing the pre-mixing/buffering method reestablish reference ranges based on their own observations. In the meantime, clinicians should be mindful of how these different AMH testing methods can substantially alter reported values. Consideration should be given to which AMH assay technique is being used before developing clinical treatment strategies for IVF patients.
References
- Durlinger AL, Visser JA, Themmen AP (2002) Regulation of ovarian function: the role of anti-Müllerian hormone. Reproduction 124(5): 601-9.
- di Clemente N, Josso N, Gouédard L, Belville C (2003) Components of the anti-Müllerian hormone signaling pathway in gonads. Mol Cell Endocrinol 211: 9-14.
- Pask AJ, Whitworth DJ, Mao CA, Wei KJ, Sankovic N, et al. (2004) Marsupial anti-Mullerian hormone gene structure, regulatory elements, and expression. BiolReprod 70: 160-7.
- van Rooij IA, Broekmans FJ, Scheffer GJ, Looman CW, Habbema JD, et al. (2005) Serum antimullerian hormone levels best reflect the reproductive decline with age in normal women with proven fertility: a longitudinal study. FertilSteril 83: 979-87.
- La Marca A, Stabile G, Artenisio AC, Volpe A (2006) Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod 21: 3103-7.
- Liberty G, Ben-Chetrit A, Margalioth EJ, Hyman JH, Galoyan N, et al. (2010) Does estrogen directly modulate anti-müllerian hormone secretion in women? FertilSteril 94: 2253-6.
- La Marca A, De Leo V, Giulini S, Orvieto R, Malmusi S, et al. (2005) Anti-Mullerian hormone in premenopausal women and after spontaneous or surgically induced menopause. J SocGynecolInvestig 12: 545-8.
- Mohamed KA, Davies WA, Lashen H (2006) Antimüllerian hormone and pituitary gland activity after prolonged down-regulation with goserelin acetate. FertilSteril 86: 1515-7.
- Visser J. (2006) Role of anti-Müllerian hormone in follicle recruitment and maturation. J GynecolObstetBiolReprod (Paris) 35(5Ptof 2): 2S30-4.
- Sills ES, Alper MM, Walsh AP (2009) Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J ObstetGynecolReprodBiol 146: 30-6.
- Han X, McShane M, Sahertian R, White C, Ledger W (2014) Pre-mixing serum samples with assay buffer is a prerequisite for reproducible anti-Mullerian hormone measurement using the Beckman Coulter Gen II assay. Hum Reprod 29: 1042-8.
- Al-Qahtani A, Muttukrishna S, Appasamy M, Johns J, Cranfield M, et al. (2005) Development of a sensitive enzyme immunoassay for anti-Mullerian hormone and the evaluation of potential clinical applications in males and females. ClinEndocrinol (Oxf) 63: 267-73.
- Nelson SM, La Marca A (2011) The journey from the old to the new AMH assay: how to avoid getting lost in the values. Reprod Biomed Online 23: 411-20.
- Rustamov O, Smith A, Roberts SA, Yates AP, Fitzgerald C, et al. (2012) Anti-Mullerian hormone: poor assay reproducibility in a large cohort of subjects suggests sample instability. Hum Reprod27: 3085-91.
- Usta T, Oral E (2012) Is the measurement of anti-Müllerian hormone essential? CurrOpinObstetGynecol 24(3): 151-7.
- Dewailly D, Andersen CY, Balen A, Broekmans F, Dilaver N, et al. (2014) The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update20(3): 370-85.
- Fleming R, Fairbairn C, Blaney C, Lucas D, Gaudoin M (2013) Stability of AMH measurement in blood and avoidance of proteolytic changes. Reprod Biomed Online 26: 130-2.
- Bonifacio M, Bradley CK, Karia S, Livingstone M, Bowman MC, etal. (2015) The original Beckman Coulter Generation II assay significantly underestimates AMH levels compared with the revised protocol. J Assist Reprod Genet PMID: 26466940.
- Clark CA, Laskin CA, Cadesky K (2014) Anti-Mullerian hormone: reality check. Hum Reprod29: 184-5.
- Wallace A, Faye SA, Fleming R, Nelson SM (2011) A multicentre evaluation of the new Beckman Coulter anti-Mullerian hormone immunoassay (AMH Gen II).Ann ClinBiochem48(Pt 4): 370-3.
- Castro C, Gourley M (2010) Diagnostic testing and interpretation of tests for autoimmunity. J Allergy ClinImmunol 125(2Suppl2): S238-47.
- Chen M, Daha MR, Kallenberg CG (2010) The complement system in systemic autoimmune disease. J Autoimmun 34: 276-86.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences