Netrin-4 Expression and Regulation by Hypoxia in Human Placenta
M Dakouane-Giudicelli, M Benharouga, N Alfaidy and P de Mazancourt
Mbarka Dakouane-Giudicelli1*, P de Mazancourt1,2,3, N Alfaidy4,5,6 and M Benharouga5,6
1Institut National de la Santé et de la Recherche Médicale, Unité 1179, Montigny Le Bretonneux, France
2Université de Versailles Saint Quentin en Yvelines, France
3AP-HP Hospital Ambroise Paré, Boulogne-Billancourt, France
4Institut National de la Santé et de la Recherche Médicale, Unité 1036, Grenoble, France
5Université. Grenoble-Alpes, 38000, Grenoble, France
6Commissariat à l’Energie Atomique (CEA), iRTSV-Biology of Cancer and infection, Grenoble, France
- *Corresponding Author:
- Mbarka Dakouane-Giudicelli
Institut National de la Santé et de la Recherche Médicale
Unité 1179, Montigny Le Bretonneux, France
Tel: +33(0)170429387
E-mail: mbarka.dakouane-giudicelli@uvsq.fr
Received date: November 01, 2017; Accepted date: November 23, 2017; Published date: November 28, 2017
Citation: Giudicelli MD, Mazancourt PD, Alfaidy N, Benharouga M (2017) Netrin-4 Expression and Regulation by Hypoxia in Human Placenta. J Reproductive Endocrinol Infert Vol.2:25. doi: 10.21767/2476-2008.100025
Copyright: © 2017 Giudicelli MD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Netrin-4 is a well-known actor involved in axonal guidance. Recently we have reported the cellular localization of netrin-4 in human placenta. Furthermore we demonstrated its role in placental angiogenesis.
Here we determined netrin-4 mRNA expression in first trimester placenta. First, we showed an increase in netrin-4 mRNA expression at 11-13 weeks of gestation (wg), suggesting a negative regulation of netrin-4 expression by hypoxia. In fact, the human placenta undergoes a transition from a low oxygenated to a highly oxygenated environment during the first trimester of pregnancy. This physiological switch in oxygen tension is a prerequisite for proper placental development and involves the hypoxia-inducible factor (HIF-1), a protein that is up-regulated under hypoxic conditions. Then, we used the trophoblast cell line BeWo to test this hypothesis. BeWo cells cultured under hypoxic conditions exhibited decreased levels of netrin-4 mRNA expression. Using siRNA strategy, hypoxia-inducible factor 1α (HIF-1α) knock-down induced a significant increase in netrin-4 mRNA expression. Altogether these results indicate that netrin-4 expression in the trophoblast cells is regulated by hypoxia via the HIF-1 pathway.
Keywords
Netrin-4; Hypoxia; cytotrophoblast; human placenta; BeWo cells
Introduction
Netrin-4 is one of the most extensively studied members of the netrin family. Netrin-4 is a secreted protein involved in neurite growth and migration orientation during the development of the central nervous system [1]. Beside the central nervous system, netrin-4 has been shown to regulate epithelial branching and morphogenesis in the lung [2], pancreas [3], salivary gland branching [4], lymphangiogenesis, angiogenesis, and tumour growth [5]. We have recently investigated and characterized the expression of netrin-4 in the human placenta [6] and determined its role in placental angiogenesis [7].
During the first trimester of pregnancy, human placenta undergoes a transition from a low oxygenated to a highly oxygenated environment. This physiological switch in oxygen tension is a prerequisite for proper placental development and involves the hypoxia-inducible factor (HIF-1), a protein that is up-regulated under hypoxic conditions. HIF-1 modulates gene transcription by binding to a specific DNA sequence known as the hypoxic response element (HRE). HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits. HIF-1β is generally constitutively expressed and insensitive to changes in O2 availability, whereas HIF-1α is acutely regulated in response to hypoxia [8].
Before 11 weeks of gestation (wg), placental oxygen remains low and is equivalent to 2-3%, which appears to be necessary to allow for specific placental metabolic activities, and to protect both placental and fetal tissues against toxic oxygen metabolites [9,10]. An increase in the oxygen level occur around 10 to 12 wg [11], when a continuous maternal blood flow is established in the intervillous space.
In the present study we investigated the effect of hypoxia on the expression of netrin-4 in isolated cytotrophoblasts from human first trimester placenta and direct involvement of HIF-1 signaling pathway in BeWo cells.
Materials and Methods
Tissues collection
Placental collection was approved by the district and local hospital ethical committees, and performed according to the Poissy Hospital code of practice. All subjects gave written informed consent. For the ontogeny study nine placentas were obtained from healthy women with viable singleton ultrasound-dated pregnancies, who were undergoing an elective termination of pregnancy procedure at first trimester between 7 and 13 wg.
Cytotrophoblast isolation: Isolation and treatment of cytotrophoblasts were performed as described by Dakouane- Giudicelli and collaborators [12]. Then cytotrophoblasts were RNA extracted. Culture of choriocarcinoma BeWo cell line under hypoxic and normoxic conditions and Invalidation of HIF-1α.
BeWo choriocarcinoma cells (ATCC Rockville, MD, USA) were plated at a density of 2.105 cells on a 35 mm dish in 2 ml DMEM/F-12 Ham (Sigma-Aldrich) containing 15% fetal bovine serum, 100 IU penicillin and 10 mg/mL streptomycin. After 24 h, cells were cultured under normoxic (20% O2) or hypoxic (2% O2) conditions for 6, 24 or 48 h.
HIF-1α knockdown induced by small interfering RNA (siRNA) in choriocarcinoma BeWo cell line, 2.105 BeWo cells were plated in a 35 mm dish in 2 ml DMEM/F-12 Ham containing 15% fetal bovine serum without antibiotics. Cells were grown up to 40– 50% confluency and then transfected with HIF-1α specific siRNA oligonucleotides (Santa Cruz biotechnology, Santa Cruz CA, USA) or with fluorescent-labeled negative control siRNAs, using Lipofectamine™ RNAiMAX (Invitrogen Carlsbad, CA, USA) for 24 h, 48 h and 72 h. Then BeWo were RNA extracted.
RNA isolation and RT-PCR analysis
RNA extraction, RNA quantification and reverse Transcription were performed, followed by quantitative PCR of duplicate sample, as previously described by Dakouane-Giudicelli et al. [12] using syber green detection on a light cycler 480 (Roche Light Cycler 480, Mannheim, Germany). The primer pair used (5′-ggcctggaagatgatgttgt-3′ and 5′-ttgaggctcttcgttcaggt-3′ amplified a 235-bp fragment of netrin-4 cDNA.
Netrin-4 mRNA relative quantification using the 2-ΔΔCp method relative to level of mean Cp values of both housekeeping genes tbp and β2m in villous cytotrophoblast cells isolated from first trimester placenta, in BeWo cells cultured under conditions of normoxia and in HIF-1α
Knock down induced by small interfering RNA (siRNA) in choriocarcinoma BeWo cell line (see table for primer reference).
| Gene | Accession number and location | Primer | Sequence | Product size (base pair) |
Tm°C |
|---|---|---|---|---|---|
| Netrin-4 | NM_021229 954-1188 |
Forward Reverse |
GGCCTGGAAGATGATGTTGT TTGAGGCTCTTCGTTCAGGT |
235 | 60 |
| HIF-1α | NM_181054.2 815-1065 |
Forward Reverse |
TCCATGTGACCATGAGGAAA CCAAGCAGGTCATAGGTGGT |
251 | 58 |
| TBP | NM_003194.3 893-1024 |
Forward Reverse |
TGACAGGAGCCAAGAGTGAA CACATCACAGCTCCCCACCA |
132 | 60 |
| B2M | NM_004048.2 589-674 |
Forward Reverse |
TGCTGTCTCCATGTTTGATGTATCT TCTCTGCTCCCCACCTCTAAGT |
86 | 60 |
Table 1: primer sequences
Statistics
All statistical analyses were performed using SigmaStat (Jandel Scientific Software, San Rafael, CA, USA). Data were analyzed by Student’s t-test
Results and Discussion
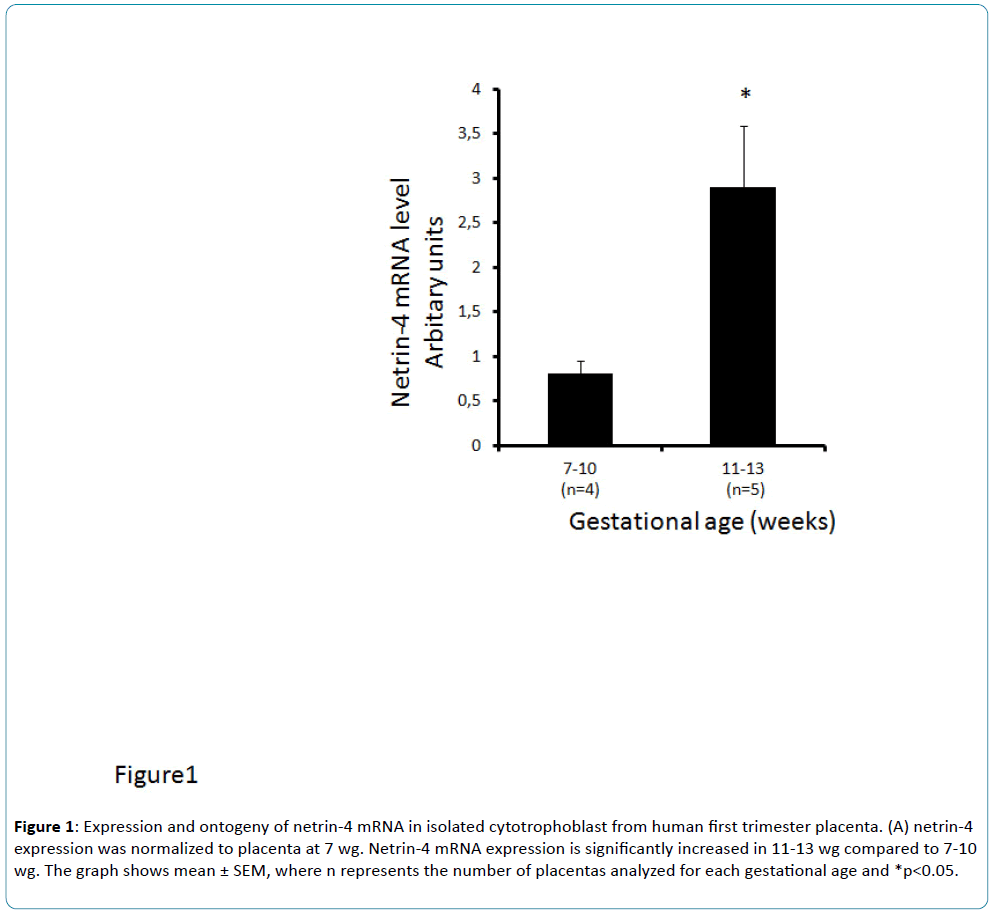
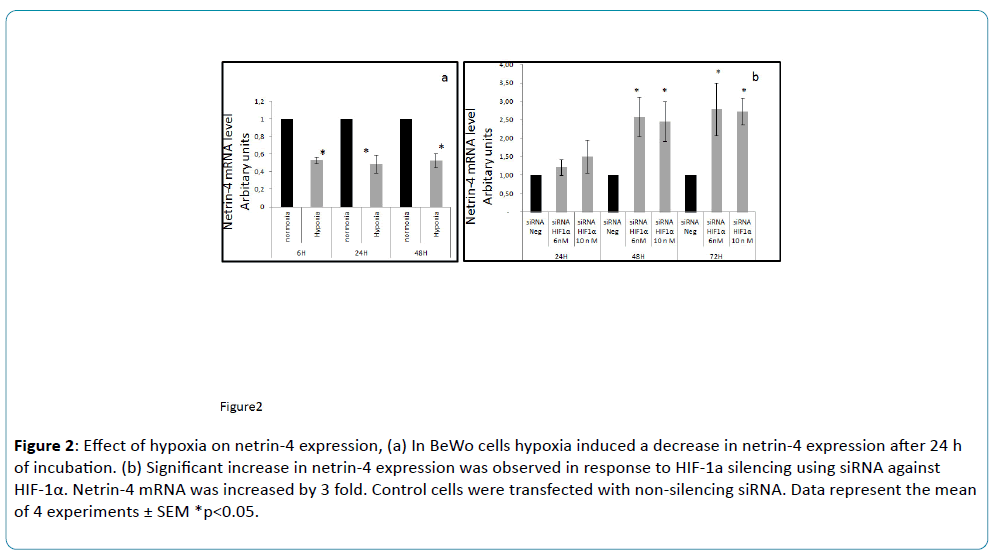
Netrin-4 mRNA expression in human placenta was low around 7-10 wg, during the gestational period of low oxygen tension, and then increased at 11-13 wg (Figure 1), when the blood flow is established and oxygen tension increases in the intervillous space. The present findings also demonstrate for the first time that netrin-4 expression is down-regulated by hypoxia in cultured BeWo cells. BeWo cells cultured under hypoxic conditions exhibited significant low levels of netrin-4 transcript when compared to normoxia (Figure 2a). Our in vitro experiments established that hypoxia is an inhibitor of netrin-4 expression. Here, we demonstrated in the human placenta a similar pattern of netrin-4 gene expression with a dependence on oxygen tension throughout the first trimester of pregnancy. We found that the changes in netrin-4 expression correlate with the changes in oxygen tension.
Figure 1: Expression and ontogeny of netrin-4 mRNA in isolated cytotrophoblast from human first trimester placenta. (A) netrin-4 expression was normalized to placenta at 7 wg. Netrin-4 mRNA expression is significantly increased in 11-13 wg compared to 7-10 wg. The graph shows mean ± SEM, where n represents the number of placentas analyzed for each gestational age and *p<0.05.
Figure 2: Effect of hypoxia on netrin-4 expression, (a) In BeWo cells hypoxia induced a decrease in netrin-4 expression after 24 h of incubation. (b) Significant increase in netrin-4 expression was observed in response to HIF-1a silencing using siRNA against HIF-1α. Netrin-4 mRNA was increased by 3 fold. Control cells were transfected with non-silencing siRNA. Data represent the mean of 4 experiments ± SEM *p<0.05.
Importantly, we also found a negative correlation between the rates of expression of netrin-4 and netrin-1 [13]. These data suggest a strong resemblance between villous cytotrophoblast from early first trimester placenta and solid tumors in terms of netrin-1 and netrin-4 transcriptional activities [14]. In the placenta, the angiogenic activity is known to be increased before its oxygenation, which correlates with the decrease in netrin-4 expression. The increase in netrin-4 levels coincides with the oxygenation of the placenta [15]. This suggests a potential role of netrin-4 in the control of angiogenic processes as demonstrated in our recent study [7].
In this work, we also demonstrated that a knockdown of HIF-1α using a siRNA strategy under hypoxic environment induced an increase in netrin-4 mRNA expression (figure 2b), providing evidence that netrin-4 down-regulation is mediated through the HIF-1 pathway. Therefore, additional research may provide new insights into the role of netrin-4 in normal human placenta and associated diseases, such as preeclampsia and growth restriction, which are both characterized by hypoxia [16].
In conclusion, our results elucidate the pattern of expression of netrin-4 and demonstrate for the first time that netrin-4 expression is down-regulated by hypoxia in the human placenta during the first trimester of pregnancy, and that its regulation is under the direct control of HIF-1. These data bring new insights into the control of Netrin-4 in the placenta and report a new mechanism for the hypoxic inactivation of netrin-4 through the HIF-1 pathway.
References
- Koch M, Murrell JR, Hunter DD, Olson PF, Jin W, et al. (2000) A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: identification, expression, and functional characterization. J Cell Biol 151: 221–234.
- Liu G, Beggs H, Jurgensen C, Park HT, Tang H, et al. (2004) Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci 7: 1222–1232.
- Yang YH, Szabat M, Bragagnini C, Kott K, Helgason CD, et al. (2011) Paracrine signalling loops in adult human and mouse pancreatic islets: netrins modulate beta cell apoptosis signalling via dependence receptors. Diabetologia 54: 828–842.
- Schneiders FI, Maertens B, Bose K, Li Y, Brunken WJ, et al. (2007) Binding of netrin-4 to laminin short arms regulates basement membrane assembly. J Biol Chem 282: 23750–23758.
- Larrieu-Lahargue F, Welm AL, Thomas KR, Li DY (2010) Netrin-4 induces lymphangiogenesis in vivo. Blood 115: 5418–5426.
- Dakouane-Giudicelli M, Duboucher C, Fortemps J, Salama S, Brule A, et al. (2012) Identification and localization of netrin-4 and neogenin in human first trimester and term placenta. Placenta 33: 677–681.
- Dakouane-Giudicelli M, Brouillet S, Traboulsi W, Torre A, Vallat G, et al. (2015) Inhibition of human placental endothelial cell proliferation and angiogenesis by netrin-4. Placenta 36: 1260–1265.
- Ozaki H, Yu AY, Della N, Ozaki K, Luna JD, et al. (1999) Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci 40: 182–189.
- Jauniaux E, Watson A, Burton G (2001) Evaluation of respiratory gases and acid-base gradients in human fetal fluids and uteroplacental tissue between 7 and 16 weeks’ gestation. Am J Obstet Gynecol 184: 998–1003.
- Illsley NP, Caniggia I, Zamudio S (2010) Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth? Int J Dev Biol 54: 409–419.
- Rodesch F, Simon P, Donner C, Jauniaux E (1992) Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstet Gynecol 80: 283–285.
- Dakouane-Giudicelli M, Duboucher C, Fortemps J, Missey-Kolb H, Brule D, et al. (2010) Characterization and expression of netrin-1 and its receptors UNC5B and DCC in human placenta. J Histochem Cytochem 58: 73–82.
- Dakouane-Giudicelli M, Alfaidy N, Bayle P, Tassin de Nonneville A, et al. (2011) Hypoxia-inducible factor 1 controls the expression of the uncoordinated-5-B receptor, but not of netrin-1, in first trimester human placenta. Int J Dev Biol 55: 981–987.
- Eveno C, Broqueres-You D, Feron JG, Rampanou A, et al. (2011) Netrin-4 delays colorectal cancer carcinomatosis by inhibiting tumor angiogenesis. Am J Pathol 178: 1861–1869.
- Kingdom JC, Kaufmann P (1999) Oxygen and placental vascular development. Adv Exp Med Biol 474: 259–275.
- Tal R, Shaish A, Barshack I, Polak-Charcon S, Afek A, et al. (2010) Effects of Hypoxia-Inducible Factor-1α Overexpression in Pregnant Mice. Am J Pathol 177: 2950–2962.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences