Antiovarian Antibody may be Used as A Predictor for Poor In Vitro Fertilization Outcome
Basak Guler, Nezaket Kadioglu, Sibel Ozler, Esma Sarikaya, Mahmut Nedim Cicek, Ali Ergun and Sertac Batioglu
1Department of Obstetrics and Gynecology, Liv Hospital, 8th Bestekar Street, Çankaya, Ankara, Turkey
2Department of Obstetrics and Gynecology, Sereflikoçhisar Government Hospital, Ankara, Turkey
3Department of Obstetrics and Gynecology, Dr. Zekai Tahir Burak Women Health and Research Hospital, Ankara, Turkey
4Department of Obstetrics and Gynecology, Kolan Hospital, Istanbul, Turkey
- *Corresponding Author:
- Basak Güler
Department of Obstetrics and Gynecology
Liv Hospital, 8th Bestekar Street
Çankaya, Ankara, Turkey
Tel: +90 5326205292
E-mail: basakgumusbg@yahoo.com
Received date: March 28, 2016; Accepted date: May 07, 2016; Published date: May 09, 2016
Citation: Güler B, et al. Antiovarian Antibody may be Used as A Predictor for Poor In Vitro Fertilization Outcome. J Reproductive Endocrinol& Infert. 2016, 1:9. doi: 10.4172/JREI.100009
Copyright: © 2016 Güler B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: The aim of this study is to demonstrate if there is any difference in the presence of antiovarian antibody (AOA) in normal fertile women and the ones having poor ovarian response and to examine if serum AOA levels could be used as a marker to detect patients at risk for poor ovarian reserve. Materials and Methods: 51 premature ovarian insufficiency (POI) patients, 47 poor responder (POR) patients to in vitro fertilization (IVF) and 51 healthy controls were included in the study. The patients were examined for their basal hormone values and antral follicle counts. Venous blood sampling for the detection of AOA was applied and ELISA technique was used to detect AOA concentrations. Results: The AOA levels were detected as 7.91 ± 1.11 U/mL in POI group, 5.72 ± 1.00 U/mL in the fertile group and 8.30 ± 0.75 U/mL in the POR group. It was significantly elevated in the POI and POR groups when compared to the fertile group (p< 0.001). There was not any difference between the AOA levels of POI and POR groups (p=0.053). The cut off levels of AOA for diagnosis of POI was detected to be 6.58 U/mL and for POR it was 7.78 U/mL. When these cut off levels were used, the sensitivity ratio was calculated as 90.2% for POI, and 94.74% for POR groups; whereas the specificity was 86.27% for POI and 83.05% for POR groups. In multivariate regression analysis of the POR group, AOA level was demonstrated to be the only significant independent variable in predicting the patients. Conclusion: Antiovarian autoimmunity plays a role in the etiopathogenesis of POI and POR; but the exact mechanism still needs to be elucidated with more clinical trials. AOA can be used as serum marker to detect the POR patients and predict IVF results. Immunosuppressive treatment of these patients may enhance the pregnancy outcomes.
Keywords
Antiovarian antibody; Premature ovarian insufficiency; IVF; Poor responder
Abbreviations
AFC: Antral Follicle Count; AOA: Antiovarian Antibody; AUC: Area Under the Curve; BMI: Body Mass Index; E2: Estradiol; FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; IVF: In Vitro Fertilization; OD: Optic Density; POI: Premature Ovarian Insufficiency; POR: Poor Responder; PRL: Prolactin; ROC: Receiver Operating Characteristic; SD: Standard Deviation; TSH: Thyroid Stimulating Hormone
Introduction
The role of autoimmunity in ovarian pathologies has been investigated in some studies [1,2]. The exact mechanism of autoimmunity playing role in the pathogenesis of idiopathic infertility, premature ovarian insufficiency (POI) and in poor responders (POR) to in vitrofertilization (IVF) has not been clearly elucidated, yet. Especially, antiovarian antibody (AOA), has been suspected to be a significant marker of the autoimmunity existing in the pathogenesis of POI and POR [3-5]. It was also shown to play role in the mechanism causing polycystic ovary syndrome, unexplained infertility and endometriosis, developing in the autoimmunity background [6].
AOA positivity was observed in 0-4% of normal population and, it was observed more in the patients of POI and POR to IVF [7]. The circulating AOA together with lymphocytic infiltrates in the ovaries were demonstrated in primary ovarian insufficiency patients and; an immune mechanism causing the situation was proposed [8]. Ovarian dysfunction is commonly related to autoimmune endocrine diseases like Hashimoto’s disease, Graves’ disease, and Addison’s disease; and nonendocrine autoimmune diseases like, systemic lupus erythematosus [9,10]. Therefore, AOA may be a demonstrator of ovarian autoimmunity in the patients ovarian dysfunction; beside of the other specific antibodies to other autoimmune diseases. So, it is possible for AOA to be used in the prediction of ovarian insufficiency in unexplained infertility patients [11,12].
Our aim in this study was to demonstrate if there was any difference in the presence of AOA in the normal fertile women and the ones having poor ovarian response and to examine if serum AOA levels could be used as a marker to detect patients at risk for poor ovarian reserve.
Materials and Methods
51 POI and 47 POR patients, who admitted to infertility clinic of Dr. Zekai Tahir Burak Women Health Education and Research Hospital, were included in the study group. 51 healthy controls, who admitted to the gynecology clinic of the same hospital, were included as the control group. All of the patients were homogenized for their ages and body mass indices (BMI).
The patients having oligo menorrhea and/or amenorrhea at least for four months and having follicle stimulating hormone (FSH) levels above 25 IU/L twice in a time period longer than four weeks, were accepted as POI [1]. POR criteria were as follows:
Advanced maternal age (above 40 years) or another risk factor like developing ovarian insufficiency after twice of maximum stimulation,
Previous experience of POR (3 or less oocytes with the conventional stimulation protocols),
Abnormal ovarian reserve (i.e. less than 5-7 antral follicle count (AFC), or antimullerian hormone level less than 0.5-1.1 ng/mL). The patients having 2 of these 3 criteria were accepted as POR [13].
The patients having menstrual periods longer than 45 days, or having 8 or less cycles in a year were defined as oligomenorrheic and the ones which did not have mensturation at least for 3 months were defined as amenorrheic [14]. The patients, who had proved their fertility with at least one delivery, and admitted to the family planning unit to obtain a birth control method during their menstrual days, were included in the control group.
The patients having any chromosomal abnormality, pregnancy, any chronic medical condition (i.e. kidney disease, diabetes, hypertension, metabolic syndrome, Cushing’s syndrome, androgen secreting tumors, late onset 21- hydroxylase deficiency, hyperprolactinemia, polycystic ovary syndrome, endometrioma), and using pills for these diseases, smoking , older than 40 years of age and having previous ovarian surgery were excluded.
The committee of ethics of Dr. Zekai Tahir Burak Women Health Education and Research Hospital had approved the study protocol, and all of the patients included in the research signed their informed consents.
All of the patients of the study were studied in their early follicular phase, at the third day of their spontaneous menstrual cycles. The patients did not have any ovulation induction treatment with clomiphene citrate or gonadotropins, before venous sampling. FSH, luteinizing hormone (LH), estradiol (E2), prolactin (PRL), and thyroid stimulating hormone (TSH) levels were measured using UniCel DxI 800 Immunoassay System (Beckman Coulter, Fullerton, CA, USA). Ovaries were examined with 7 MHz transvaginal ultrasound convex transducer (SSD 1000; Aloka, Tokyo, Japan). BMI were calculated with the formula, weight (kg)/square of height (m2).
The venous blood samples of all of the patients were centrifuged at 5000 revolution per minute (2236 x g) within one hour and stored at -80°C, to be studied all together.
The presence of ovarian autoantibodies was evaluated by the enzyme-linked immunosorbent assay (ELISA) technique. The AOA ELISA Ig-classifying test used is a solid-phase sandwich enzyme immunoassay for the quantitative determination of AOA in human serum by optical density results, from Bioserv Diagnostics (Rostock, Germany).
The ELISA plate was coated with a mixture of ovarian proteins used as capture antigens for AOA. The samples and controls were pipetted into the wells and then incubated. During incubation, AOA were bound to the antigen and immobilized on the plate. A conjugate consisting of antibodies against different regions of different classes of human immunoglobulin (IgA, IgG, IgM) was bound to the antigen– antibody complex during incubation. After removal of the unbound conjugate by washing with horseradish peroxidase, the substrate 3, 30, 5, 50-tetramethylbenzidine was added, yielding a color reaction that was stopped with 0.25 M of sulfuric acid. The extinction was measured at a wavelength of 450 nm with a microplate reader.
We preferred to use the AOA concentration levels rather than OD levels, because of the linear relationship between concentration and OD levels.
Statistical analysis
Data analysis was performed by using SPSS for Windows, version 17 (SPSS Inc., Chicago, IL, United States). The Kolmogorov Smirnov test was used to test whether continuous variables were distributed normally or not. Homogeneity of variances was evaluated by the Levene test. Continuous variables were shown as mean ± standard deviation (SD). Mean differences between groups were compared by ANOVA test. The optimal cut-off points of the clinical measurements, which discriminated groups from each other, were evaluated by receiver operating characteristic (ROC) analysis calculating area under the curve (AUC) as giving the maximum sum of sensitivity and specificity for the significant test. Sensitivity, specificity, positive and negative predictive values were also calculated at the best cut-off point for each clinical measurement. The best predictors which discriminated groups from each other were determined by multiple logistic regression analysis, where applicable. Any variable, whose univariate test had a p-value<0.05 was accepted as a candidate for the multivariable model, along with all variables of known clinical importance. Adjusted odds ratios, 95% confidence intervals and Wald statistics were calculated for each variable. Any p-value less than 0.05 were considered statistically significant.
Results
149 patients, including 51 POI, 47 POR patients having IVF treatment because of poor ovarian capacity, and 51 healthy controls, were studied. The groups were homogenized for their ages and BMI. The clinical, ultrasonographic and andropometric parameters of the groups were demonstrated in Table 1. 27 patients (52.9%) of the POI group and only 1 patient (2.2%) of the POR group were amenorrheic, whereas fertile group did not possess any amenorrheic patients. This difference was significant when the POI and POR groups were compared with the fertile group individually (p<0.001 and p<0.001, respectively). Having AFC less than 8 was present in 47 patients (92.2%) of POI group, 24 patients (52.2%) of POR group and 5 patients (9.8%) of the fertile group; which was significantly different (p<0.001 and p<0.001) (Table 1).
| GROUP 1 (POF) N=51 | GROUP 2 (CONTROL) N=51 | GROUP 3 (POR) N=47 | p* value | p** value | |
|---|---|---|---|---|---|
| Age* | 31.82±5.57 | 31.39±5. 36 | 32.39 ±4.76 | P(1-2)=0.621 | 0.523 |
| P(2-3)=0.756 | |||||
| P(1-3)=0.596 | |||||
| BMI * | 29.89 ± 3.82 | 29.03 ± 3.66 | 28.87 ± 4.12 | P(1-2)=0.769 | 0.07 |
| P(2-3)=0.069 | |||||
| P(1-3)=0.082 | |||||
| Amenorrhea ** | 27(52.9%) | 0(0%) | 1(22%) | P(1-2)<0.001 | <0.001 |
| P(2-3)=0.474 | |||||
| P(1-3)<0.001 | |||||
| POF in the mother ** | 5(9.8%) | 6(11.8%) | 3(6.5%) | P(1-2)=0.500 | 0.675 |
| P(2-3)=0.298 | |||||
| P(1-3)=0.417 | |||||
| POF in the sister ** | 6(11.8%) | 1(2.0%) | 1(2.2%) | P(1-2)= 0.032 | 0.046 |
| P(2-3)=0.726 | |||||
| P(1-3)=0.044 | |||||
| AFC < 8** | 47(92.2%) | 5(9.8%) | 24(52.2%) | P(1-2)<0.001 | <0.001 |
| P(2-3)=0.001 | |||||
| P(1-3)<0.001 |
Table 1: Comparison of clinical, ultrasonographic and andropometric parameters of the groups (*Independent simple t test, **Pearson's chi-square test. p value; within groups, ** p value between groups. POF: Premature Ovarian Failure; POR: Poor Responder; AFC: Antral Follicle Count; BMI: Body Mass Index).
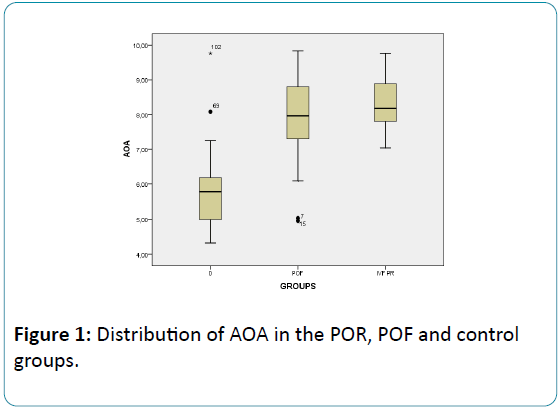
The mean level of AOA was detected as 7.91 ± 1.11 U/mL in the POI group; 8.30 ± 0.75 in the POR group and 5.72 ± 1.00 in the fertile group. AOA was found significantly elevated in the POI and POR groups than the control group (p<0.001 and p<0.001, respectively), but did not differ when the two were compared (p=0.053) (Table 2, Figure 1).
| GROUP 1 (POF) N= 51 | GROUP 2 (CONTROL) N= 51 | GROUP 3 (POR) N= 47 | P* VALUE WITH IN GROUPS | P** VALUE BETWEEN GROUPS | |
|---|---|---|---|---|---|
| FSH (mIU/ml) | 46.43 ±28.28 | 6.99 ±2.00 | 7.06 ±2.36 | P(1-2)<0.001 | <0.001 |
| P(2-3)=0.983 | |||||
| P(1-3)<0.001 | |||||
| LH (mIU/ml) | 27.73 ±20.73 | 4.80 ±2.74 | 4.45 ±2.15 | P(1-2)<0.001 | <0.001 |
| P(2-3)=0.888 | |||||
| P(1-3)<0.001 | |||||
| E2 (pmol/l) | 41.41 ±30.03 | 50.62 ±27.62 | 59.92 ±21.08 | P(1-2)=0.083 | 0.004 |
| P(2-3)=0.089 | |||||
| P(1-3)=0.001 | |||||
| PRL (ng/ml) | 11.35 ±8.48 | 11.42 ±9.22 | 15.57 ±8.29 | P(1-2)=0.971 | 0.048 |
| P(2-3)=0.033 | |||||
| P(1-3)=0.030 | |||||
| TSH (uIU/ml) | 2.15 ± 1.44 | 1.88 ±1.24 | 2.66 ±1.80 | P(1-2)=0.354 | 0.036 |
| P(2-3)=0.011 | |||||
| P(1-3)=0.096 | |||||
| AOA (U/Ml) | 7.91±1.11 | 5.72 ±1.00 | 8.30 ±0.75 | P(1-2)<0.001 | <0.001 |
| P(2-3)<0.001 | |||||
| P(1-3)=0.053 |
Table 2: Comparison of hormonal parameters and AOA among groups (FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; E2: Estradiol; PRL: Prolactin; TSH: Thyroid Stimulating Hormone; AOA: Anti Ovarian Antibody).
FSH and LH levels in the patients were significantly higher in POI group than the control and POR group (p<0.001 and p<0.001, respectively). E2 levels in the POR group were higher when compared to the POI group (p<0.001); but did not differ from the E2 levels of the control group (p=0.089). PRL was significantly higher in the POR group than the other two groups (p=0.030 and p=0.033, respectively) (Table 2).
ROC curve analysis was applied for the POI and POR groups. 6.58 U/mL and 7.78 U/mL were defined as cut off levels for serum AOA for the POI and POR groups, respectively [area under curve (95% CI); 0.926(0.867-0.985) and 0.782 (0.711-0.853)]. These cut off levels led to sensitivity ratios of 90.20% and 94.74% for the POI and POR groups, respectively; whereas the specificity ratios were calculated as 86.27%, and 83.05%, respectively (Table 3).
| AOA | Cut off (U/ml) | Sen(%) | Spe (%) | Positive predictivity (%) | Negative predictivity (%) | Area under the curve (95%Cl) | P value |
|---|---|---|---|---|---|---|---|
| POF | 6.58 | 90.20% | 86.27% | 86.79% | 89.80% | 0.926(0.867-0.985) | <0.001 |
| POR | 7.78 | 94.74% | 83.05% | 78.26% | 96.08% | 0.782 (0.711-0.853) | <0.001 |
Table 3: AOA cut off levels, sensitivity and specificity ratios and predictive values for the POF and POR groups (POF: Premature Ovarian Failure; POR: Poor Responder; AOA: Anti Ovarian Antibody).
Multiple regression analysis was used to calculate the predictive independent variables in detecting increased POR risk. Above the AOA cut off level (≥ 7.780 U/ml) with odds ratio: 13.978, 95% confidence interval Cl: 4.383-44.573 was detected and AOA was calculated to be the only significant independent variable in predicting POR patients (p<0.001) (Table 4). Age and AFC less than 8 was found to be related to POR, in univariate analysis, whereas in multivariate analysis, they were not related to POR risk prediction (p=0.121 and p=0.261, respectively) (Table 4).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| AUC (95%Cl) | p value | AUC (95%Cl) | p value | |
| Age | 1.123 (1.033-1.220) | 0.006 | 1.174 (0.959-1.437) | 0.121 |
| Irregular menses | 1.093 (0.310-3.864) | 0.89 | ||
| POF experienced mother | 0.523 (0.123-2.225) | 0.381 | ||
| POF experienced sister | 1.111 (0.068-18.289) | 0.941 | ||
| Presence of autoimmune disease | 0.516 (0.156-1.709) | 0.279 | ||
| FSH (mIU/ml) | 1.015 (0.844-1.221) | 0.872 | ||
| LH (mIU/ml) | 0.841 (0.793-1.116) | 0.484 | ||
| AFC < 8 | 0.122 (0.044-0.342) | <0.001 | 0.295 (0.035-2.478) | 0.261 |
| AOA (U/ml) | 16.489 (5.309-51.212) | <0.001 | 13.978 (4.383-44.573) | <0.001 |
Table 4: Relations of the variables with POR (FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; AFC: Antral Follicle Count; AOA: Anti Ovarian Antibody).
Multiple regression analysis was applied to define independent variables in predicting increased POI risk. Only the FSH, with OR: 1.643, 95%Cl: 1.063-2.539, was demonstrated to have positive predictive value in observing POI (p=0.025) (Table 5). Although AOA, AFC<8, LH, age and irregular menses were found related to POI in univariate analysis; they were not calculated so in multivariate analysis (Table 5).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| AUC(95%Cl) | p value | AUC(95%Cl) | p value | |
| Age | 1.085 (1.008-1.167) | 0.029 | 1.466 (0.816-2.631) | 0.2 |
| Irregular menses | 0.067 (0.020- 0.222) | <0.001 | 6.318 (0.000-30.278) | 0.737 |
| POF experienced mother | 0.815 (0.232-2.862) | 0.75 | ||
| POF experienced sister | 6.667 (0.773-57.518) | 0.084 | ||
| Presence of autoimmune disease | 0.584 (0.177-1.925) | 0.377 | ||
| FSH (mIU/ml) | 1.649 (1.265-2.150) | <0.001 | 1.643 (1.063-2.539) | 0.025 |
| LH (mIU/ml) | 1.605 (1.283-2.010) | <0.001 | 1.825 (0.887-3.754) | 0.102 |
| AFC <8 | 0.011 (0.003-0.043) | <0.001 | 6.813 (0.025-18.499) | 0.502 |
| AOA (U/ml) | 5.844 (3.050-11.196) | <0.001 | 0.947 (0.065-13.851) | 0.947 |
Table 5: Relations of the variables with POF (FSH: Follicle Stimulating Hormone; LH: Luteinizing Hormone; AFC: Antral Follicle Count; AOA: Anti Ovarian Antibody).
Discussion
In this study, we aimed to find out if AOA is predictive or not in the diagnosis of patients having poor ovarian capacity. When we compared the groups, we observed that POI and POR groups had significantly higher levels of AOA than the fertile control group; whereas they were not different from each other. We also observed decreased AFC in the POI and POR groups than the control group, as expected [15]. When the basal hormone levels were compared, FSH and LH levels were obviously higher; E2 levels were lower in the POI group than the POR and control groups. There was not a difference in hypothyroidism frequency between the groups; but the POR group had higher TSH and PRL levels than the other two; which made us think that subclinic hypothyroiditis was more frequent in the POR group.
The mechanisms of some specific antigens targeting ovaries and taking place in the development of POI, were described. Steroid cell antibodies [16], highly positive in especially Addison’s disease, 3b-hydroxysteroid dehydrogenase autoantibodies [17], and gonadotropin receptor blocking antibodies [18] are examples to these. Although the case number is limited, Damewood et al. [19] showed AOA presence in 9 of 27 POI patients, immunohistochemically; and Luborsky et al. [1] demonstrated AOA in 33 of 45 POI patients having IVF.
The researches studying antiovarian autoimmunity and POI show that genetic factors may be effective on disease pathogenesis [20]. Familial prevalence of POI varies between 4%-31%. The wide range may be caused by the changes in the definition of POI, election of the study population, and the presentation of the data [21]. In our study, having a POI experienced mother or sister prevalence in the POI group was 17.6%, whereas it was 9.8% in the control group. The incidence was suitable with the literature, but the difference between the groups was not important. Besides, the AOA concentrations of the patients who had a family member experiencing POI and who had not, did not differ in our study.
We observed that only the elevation of the levels of FSH in the POI group, and elevation in the levels of the AOA in POR group, had predictive value in the diagnosis of the diseases. Although AOA level was increased in both of the POI and POR groups, it was only predictive in the POR group.
Antiovarian autoimmunity was shown to be relevant in the ethiopathogenesis of the POR group [22,23]. Similarly, our study demonstrated the significantly higher incidence of AOA in the POR group than the control group. In rat studies, antioocyte cytoplasm antibodies in the follicle aspirates of the ovarian insufficiency group were found in elevated levels than the control group’s [24]. A negative relationship between ovarian autoimmunity and outcome of IVF attempts has been demonstrated in a number of studies, and it has been speculated that repeated IVF attempts might induce AOA [3,25-27]. Measuring AOA before the treatment period; may help to inform patients about the success of the IVF attempt [28,29]. There are also studies, showing the success of immunosuppressive treatment in obtaining pregnancy, in POR and POI patients [25,30-33].
Conclusion
The antiovarian autoimmunity plays a role in the ethiopathogenesis of POI and POR to IVF treatments; but the exact mechanism still needs to be elucidated with more clinical trials. De novo genetic anomalies (like mutation, deletion, duplication) which may cause ovulatory dysfunction, protein expression abnormalities (real-time PCR), the histone methylation mechanisms of the DNA, immune mechanisms and the biochemical changes in molecular levels must be investigated.
Our study has limitations because of small sample size; but we observed a definite difference in AOA concentrations of POR patients and the healthy, fertile controls; and detected a serum cut off level for AOA; which can be used as serum marker to detect the POR patients and predict IVF results. Besides, using some kind of steroid treatments may suppress the autoimmunity in these patients, and may help to enhance the pregnancy outcomes.
Acknowledgement
Some results of the research were presented in poster form, in ESHRE 2012.
References
- Luborsky JL, Visintin I, Boyers S, Asari T, Caldwell B, et al.(1990) Ovarian antibodies detected by immobilized antigen immunoassay in patients with premature ovarian failure. J ClinEndocrinolMetab70: 69-75.
- Pires ES, Parte PP, Meherji PK, Khan SA, Khole VV(2006) Naturally occurring anti-albumin antibodies are responsible for false positivity in diagnosis of autoimmune premature ovarian failure. J HistochemCytochem54: 397-405.
- Gobert B, Barbarino-Monnier P, Guillet-May F, Béné MC, Faure G (1992) Anti-ovary antibodies after attempts at human in-vitro fertilization induced by follicular puncture rather than hormonal stimulation. J ReprodFertil96: 213-218.
- Barbarino-Monnier P, Jouan C, Dubois M, Gobert B, Faure GC, Béné MC (2003)Antiovarian antibodies and in vitro fertilization: cause or consequence? GynécolObstétFertil31: 770-773.
- POI Guideline Development Group (2015) Management of women with premature ovarian insufficiency. Guideline of the European Society of Human Reproduction and Embryology (ESHRE).
- Luborsky J (2002) Ovarian autoimmune disease and ovarian autoantibodies. J Women's Health Gend Based Med 11: 585-599.
- Forges T, Barbarino-Monnier P, Faure GC, Bene MC (2004) Autoimmunity and antigenic targets in ovarian pathology. Hum Reprod10: 163-175.
- Coulam CB, Ryan RJ (1979) Premature menopause. I Etiology. Am J ObstetGynecol133: 639-643.
- Baker JR (1992) Immunologic aspects of endocrine diseases. JAm Med Assoc 268: 2899-2903.
- Jandreski, M (1994)Anticardiolipin antibodies and the antiphospholipid antibody syndrome. Clin. Chem. News: 8–9.
- Dragojevic-Dikic S, Marisavljevic D, Mitrovic A, Dikic S, Jovanovic T, et al. (2010) An immunological insight into premature ovarian failure (POI). Autoimmunity Reviews 9: 771-774.
- LuborskyJ, Lianes B, Davies S, Binor Z, Radwanska E, et al. (1999) Ovarian autoimmunity: greater frequency of autoantibodies in premature menopause and unexplained infertility than in the general population. ClinImmunol90: 368-374.
- Ferraretti AP, La Marca A,Fauser BCJM, Tarlatzis B, Nargund G, et al. (2011) ESHRE consensus on the definition of ‘poor response’ to ovarian stimulation for in vitro fertilization: the Bologna criteria. Human Reproduction26: 1616-1624.
- Wiksten-Almstromer M, Hirschberg AL, Hagenfeldt K (2008) Prospective follow-up of menstrual disorders in adolescence and prognostic factors. ActaObstetricia et GynecologicaScandinavica 87: 1162-1168.
- Knauff EAH, Eijkemans MJC, Lambalk CB, ten Kate-Booij MJ, Hoek A, et al. (2009) Anti-MüllerianHormone,Inhibin B andAntralFollicle Count in Young Women with Ovarian Failure. J ClinEndocrinolMetab 94: 786-792.
- Falorni A, Laureti S, Candeloro P, Perrino S, Coronella C (2002) A Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. FertilSteril 78:270-279.
- Arif S, Vallian S, Farzaneh F, Zanone MM, James SL(1996) Identification of 3 beta-hydroxysteroid dehydrogenase as a novel target of steroid cell autoantibodies: association of autoantibodies with endocrine autoimmune disease. J ClinEndocrinolMetab81; 4439-4445.
- Anasti JN, Flack MR, Froehlich J, Nelson LM (1995) The use of human recombinant gonadotropin receptors to search for immunoglobulin G mediated premature ovarian failure. J ClinEndocrinolMetab80: 824-828.
- Damewood MD, Zacur HA, Hoffman GJ, Rock JA (1986) Circulating antiovarian antibodies in premature ovarian failure. ObstetGynecol68: 850-854
- Shelling AN (2010) Premature ovarian failure. Reproduction 140: 633-641.
- van Kasteren YM, Hundscheid RDL, Smits APT, Cremers FPM, van Zonneveld P (1999) Familial idiopathic premature ovarian failure: an overrated and underestimated genetic disease? Hum Reprod 14: 2455-2459.
- Kalantaridou SN, Braddock DT, Patronas NJ, Nelson LM (1999) Treatment of autoimmune premature ovarian failure. Hum Reprod 14: 1777-1782.
- MiyakeT, Sato Y, Takeuchi S (1987) Implications of circulating auto-antibodies and peripheral blood lymphocyte subsets for the genesis of premature ovarian failure. J. Reprod. Immunol 12: 163-171
- Horejsi J, Martinek J, Novakova D, Madar J, Brandejska M (2000) Autoimmune antiovarian antibodies and their impact on the success of an in IVF/ET program. Ann NY AcadSci 900:351-356.
- Barbarino-Monnier P, Gobert B, Guillet-May F, Béné MC, Barbarino A (1995)Ovarian autoimmunity and corticotherapy in an in-vitro fertilization attempt. Hum Reprod10: 2006-2007.
- Geva E, Vardinon N, Lessing JB, Lerner-Geva L, Azem F, et al. (1996) Organ-specific autoantibodies are possible markers for reproductive failure: a prospective study in an in-vitro fertilization-embryo transfer programme. Hum Reprod11:1627-1631.
- Gobert B, Barbarino-Monnier P, Guillet-Rosso F, Béné MC, Faure GC (1990) Ovary antibodies after IVF. Lancet 335: 723.
- Khole V (2010) Does ovarian autoimmunity play a role in the pathophysiology of premature ovarian insufficiency? J Midlife Health1: 9-13.
- Bakun OV, Sekeiros Peres V, Voitko M (2014) Does ovarian autoimmunity play a role in the pathophysiology of premature ovarian insufficiency 3: 18-21.
- Cowchock FS, McCabe JL, Montgomery BB (1988) Pregnancy after corticosteroid administration in premature ovarian failure (polyglandularendocrinopathy syndrome). Am J ObstetGynecol 158: 118-119
- Blumenfeld Z, Halachmi S, Peretz BA, Shmuel Z, Golan D (1993) Premature ovarian failure. The prognostic application of autoimmunity on conception after ovulation induction. FertilSteril 59: 750-755.
- Corenblum B, Rowe T, Taylor PJ (1993) High-dose, short-term glucocorticoids for the treatment of infertility resulting from premature ovarian failure. FertilSteril 59: 988–991.
- Bateman BG, NunleyWC, Kitchin JD (1983) Reversal of apparent premature ovarian failure in a patient with myastenia gravis. FertilSteril39: 108-110.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences